b Center for Functional Nanomaterials, Brookhaven National Laboratory, Upton, NY 11973, United States;
c State Key Laboratory of Silicon Materials, Zhejiang University, Hangzhou 310027, China;
d Center of Electron Microscopy, Department of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, China
Biominerals generally exhibit superior mechanical properties over the synthetic relatively pure crystals and the improved properties are partially attributed to the biogenic composite structures where single crystals incorporate biomacromolecules to form single-crystal composites, as found in echinoderms and mollusks [1]. Biomacromolecules occluded inside the singlecrystalline matrix could distribute as micrometer-sized networks [1b, 2] as well as nanometer-sized fibers and particles [1d, 1f, 3]. The organic matrix composed of proteins and other biomacromolecules contribute to overcome the intrinsic brittleness and enhance the mechanical properties of the single crystals [1d, 1e, 4]. Furthermore, the precise control of the texture [1e], lattice strain [5], magnesium incorporation [6] and disorder [7] are also associated with the mechanisms for mechanical reinforcement.
The distinct structure and superior properties of the singlecrystal composites have lastingly spurred the efforts towards their synthetic analogs. And a large variety of the single-crystalline hosts with incorporated guest materials have been reported [8]. The guest materials, such as particles [9], micelles [8, 10], continuous networks [8e, 11], and possibly the individual molecules [8d, 12], may be introduced into single-crystalline host matrix. The accumulating examples and efforts gradually reveal the incorporation mechanisms and, equally importantly, advance the research of single-crystal composites from structural mimic to functional design, providing new sight on broadening application of these unconventional solids [8c, 8d, 11c, 12b, 13].
The incorporation of guest materials will change the properties of the host crystals. On one hand, effects of guest incorporation on the electronic properties have been found, in terms of shift in the band gap of a few semiconducting crystals [8d, 14]. On the other hand, whether or not the guest incorporation leads to the similar mechanical enhancement as in the cases of biominerals has been attracting the attention of researchers. Once the protein crystals incorporate the gel networks, the crystal composites exhibit improved mechanical properties and stability (e.g., against dehydration) that enable handling and characterization at room temperature [8a, 8b, 11b]. Apart from the protein crystals, calcite single-crystal tends to resist crack by dissipating energy after the incorporation of polymer latex particle [9b]. Also, the mechanical properties of calcite single-crystal with carbon-based nanomaterials [13b, 15], copolymer micelle [8c], amino acids [12b] or polyelectrolyte [16] incorporated have been studied with nanoindentation test. Generally, the hardness of the crystals increased [8c, 10b, 12b, 13b, 15] and in some cases, the improved hardness could even reach the values of their biogenic counterparts [12b]. The mechanism of the increased hardness might originate from local lattice strain [10b] and covalent bonds pinning [12b]. Although the mechanical property of single-crystal composites is the main research focus, its investigation methods are still very limited with nano-indentation as the dominating approach. Developing alternative nanoscale and in-situ strategy becomes highly desired. Here, transmission electron microscopy-scanning probe microscopy (TEM-SPM) platform [17] is used to examine the in situ compression test of single-crystal composites where calcite crystals are the host while agarose fibers, multi-walled carbon nanotubes (MWCNTs), and graphene oxide (GO) are selected as the guests. Visualizing the composites subject to compression shows their fracture tolerance and indicates that the guests improve the toughness of single-crystal host by inhibiting crack growth.
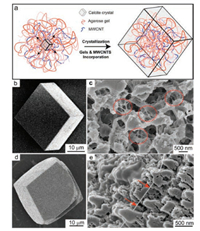
We first prepared the calcite single crystals with incorporated agarose fibers and MWCNTs by crystallization in agarose gels without or with dispersed MWCNTs using a previously-reported method depicted in Fig. 1a [8e, 8f, 8l]. Without the MWCNTs, crystals grown from agarose gels are expressed as the characteristic rhombohedral morphology of calcite single crystals (Fig. 1b) but the composite structures in the crystals can be revealed by imaging the etched surfaces of the crystals. Scanning electron microscopy (SEM) images of etched surfaces exhibit the exposed gel fibers from irregular etch pits as previous reported (Fig. 1c), demonstrating the incorporation of agarose fibers [18]. The amount of the incorporated agarose is approximately 2370ppm measured by thermogravimetric analysis (TGA) (Fig. S1 in Supporting information) [11c]. Furthermore, mechanically strong MWCNTs [19] were incorporated into the crystals. MWCNTs with carboxyl groups were well dispersed into the agarose gel before crystallization and the subsequent crystal growth resulted in calcite crystals with incorporated agarose fibers and MWCNTs (Fig. 1a) [8f]. The obtained crystals are slightly colored in grey, compared to the transparent crystals grown without MWCNTs (Fig. S2 in Supporting information). And the sharp edges of crystals turn curved because of the interaction between the carboxyl groups and the growing crystals (Fig. 1d) [9b]. Imaging the etched crystal surfaces shows the relatively rigid and isolated fibers (Fig. 1e, arrows) in addition to the agarose fibers, indicative of the MWCNT incorporation. As the morphology of MWCNT and agarose fibers are similar, the amount of MWCNT incorporated still needs further investigation.
|
Download:
|
| Fig. 1. (a) Schematic representation of crystal growth in gel media containing dispersed MWCNTs; SEM images of calcite crystals grown in agarose gels: (b) as grown crystal, (c) etched crystals where red dotted circles highlight the gel fibers; SEM images of as grown (d), etched (e) calcite crystals grown from agarose gels containing dispersed MWCNTs, with the red arrows pointing at a MWCNT. | |
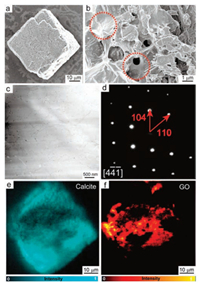
We further examined the internal structures of the obtained composite crystals using electron microscopy. Thin sections cut from calcite crystals grown in presence of MWCNTs were prepared by focused ion beam (FIB). High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) was employed to image the thin sections, showing random interconnected networks distributed throughout the thin sections which are similar to the previously-reported agarose fiber networks inside calcite crystals (Fig. 2a) [8e]. Considering the similar morphologies and contrast of agarose fibers (diameter: 13 ± 5 nm) and MWCNTs (diameter: 10–20nm, Fig. S3a in Supporting information) in the HADDF-STEM image, it is hard to conclude if the MWCNTs are incorporated together with the agarose. In order to identify the MWCNTs, the crystals were characterized by Raman intensity mapping in 1050–1150 cm-1 and 1500–1650 cm-1 (characteristic Raman shifts of calcite and MWCNTs respectively, Fig. S4 in Supporting information) [20]. We first imaged the as-grown crystals after removing the MWCNTs adsorbed on surface by sonication. According to the Raman mapping, the calcite singlecrystal was mapped clearly (Fig. S5a in Supporting information) and the MWCNTs adsorbed on the surface was almost eliminated (Fig. S5b in Supporting information). Further, we gently etched the crystal to investigate the distribution of the MWCNTs inside the crystalline matrix (Figs. 2c and d). The fire-colored mapping marks the region of MWCNTs emerging from the etched pits of crystal, indicating the existence of MWCNTs inside the crystalline matrix (Fig. 2d). The concentration of the MWCNT in the crystals is 356 ppm, as measured by Raman spectroscopy (Fig. S6 in Supporting information) [21]. The concentration of MWCNT incorporated in crystals is approximately to that dispersed in gel medium, 370 ppm, indicating an effective incorporation approach reported here. Despite the presence of the incorporated guests including agarose fiber and MWCNTs, the selected-area electron diffraction (SAED) demonstrate single-crystallinity of a large area (diameter: 3 μm), by showing a single set of diffraction spots consistent with the calcite single-crystal (Fig. 2b). Therefore, the optical microscopy (OM), SEM, SAED, Raman spectroscopy evidences demonstrated that the MWCNTs are incorporated inside the calcite single crystals, together with the agarose gel fibers.

|
Download:
|
| Fig. 2. (a) A HAADF-STEM image of a thin section cut from calcite crystals grown in agarose gels containing MWCNTs; (b) A SAED pattern of the thin section; (c-d) Raman intensity maps of an etched calcite crystal where calcite is identified (1050-1150 cm-1) in (c) and the distribution of MWCNTs is marked (1500-1650 cm-1) in (d). | |
Next, we further studied the mechanical properties of calcite single-crystals with guests incorporated. As a comparison, we prepared calcite single-crystal without agarose fibers and MWCNTs by solution grown method. We examined the toughness of the crystals by in situ nanoscale observation in TEM-SPM (Fig. S7 in Supporting information) [17]. The crystals were first prepared into nanocuboids with the rectangle contact cross-section (width: ~200 nm, thickness: 500 nm, Table S1 in Supporting information) using FIB and a constant compressed force were steadily introduced onto the nanocuboid. By recording the rupture time of the compressed nanocuboid, we could conclude the ability of damage-tolerance of each crystalline sample. Considering the different mechanical performance in different crystal-planes, three samples were measured for each crystal. The results show that the calcite single-crystal with agarose fibers incorporated exhibit stronger damage resistance than the pure counterparts, with the rupture time increasing from 12 ± 4 s to 21 ± 4 s (Fig. S8 in Supporting information). The rupture time further increases to 27 ± 3 s when the MWCNTs are introduced into the crystals as well.
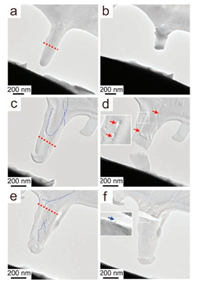
The in-situ technique we adopted here enables visualizing the deformation of composite single crystals at nanoscale, thus exhibiting advantages indepth elucidation of the mechanism for toughening single-crystal. We examine the fracture behavior of varied nanocuboids and two features are clearly seen. First, it is found that the fracture surfaces emerge at the locations without nanofibers (Figs. 3a, c, e, red dash lines) by comparing the images before and after the fracture of each sample (Fig. 3). Second, microcracks are found at the regions with lower contrast where nanofibers bridge the crack faces (Fig. 3d, arrows). These two fracture features are consistent with the toughening mechanism of crack bridging that is the primary source of toughening in brittle materials such as metals and ceramics [22]. And similar crack bridging phenomenon was observed in the fractured calcite crystals with incorporated particles[9b]. Herein, the nanofiber guest introduced inside single-crystalline hosts acts as the nanoscale fiber-like bridges across the crack and shield the crack tip, providing an origin of toughening. In addition, the crack propagation is restrained when the developing cracks meet the fiber phase. Furthermore, an even greater improvement to the single-crystal toughening occurs in the form of fiber guest pull-out together with crack bridging, as shown in Fig. 3f. According to the studies on toughening polymer [23], the pull-out process from the crystalline matrix dissipates energy through frictional sliding and leads to the formation of a crack-bridging zone near the tip, which opposes the applied stress for cracking.
|
Download:
|
| Fig. 3. TEM images of varied calcite single-crystalline cuboids before (a, c, e) and after (b, d, f) rupture. From up to down: the solution-grown calcite crystal, the gelgrown calcite crystal and the gel-grown calcite crystal with MWCNTs incorporated. The red and blue dotted lines highlight the rupture surfaces and the fibers respectively. The red arrows point at the microcrack bridging regions. The insets show subsections of the TEM images where the microcracks with lower contrast are highlighted. The blue arrow points at the pull-out fiber. | |
Apart from MWCNT, we also investigated the mechanical properties of gel-grown calcite single-crystal incorporating mechanically strong GO (Fig. S3b in Supporting information) [24]. Recently, the GO was incorporated into the solution-grown calcite single-crystals and the obtained composite crystal exhibited lower elastic modulus and higher hardness in the nanoindentation test, compared to pure calcite single-crystal [13b]. The reasons for GO incorporation were attributed to the effective interaction between GO and CaCO3 [25], as well as the sharply reduction of surface energy of GO during incorporation [26], but both the amount and the spatial distribution of the incorporated GO guest were not discussed [13b]. Instead of solution, we employed gel media for crystallization because the gel networks can confine the motion of the GO dispersed in the gels and provide an extra mechanism to favor the GO incorporation. The calcite crystals grown in GO dispersed gels are curved with slightly rough surfaces (Fig. 4a) [13b]. Gently etching the crystals reveals the heterogeneous internal structure of the crystal by exposing flake shaped materials in the etched pits, which were identified as the GO platelets (Fig. 4b). In addition to the GO, the agarose gel networks incorporated inside the crystals are shown by STEM (Fig. 4c). The composite crystals maintain the single-crystal nature demonstrated by SAED of a large area (diameter: 3 μm) (Fig. 4d). Raman analysis shows that the concentration of GO incorporated is 348ppm (Fig. S6 in Supporting information), approximate to that dispersed in gel media (370ppm), indicating an effective incorporation of GO platelets. Further, Raman intensity map (Figs. 4e, f and S4, S5c, S5d in Supporting information) [27] for the etched crystal surfaces indicates that the distribution of GO inside the crystalline matrix is not as uniform as that of MWCNTs. The non-uniform distribution is due to the partial aggregation of GO caused by the CaCl2 that destabilizes GO aggressively as previously reported [28]. Importantly, TEM-SPM study shows the rupture time of 18± 5 s that is even lower than 21± 2 s for the sample without GO incorporated (Fig. S8 in Supporting information). The reduced toughness might be due to the relatively non-uniform distribution of GO platelets.
|
Download:
|
| Fig. 4. SEM images of as grown (a), etched (b) calcite crystals grown from agarose gels containing dispersed GO, with the red dotted circles highlighting the GO platelets; (c) A HAADF-STEM image of a thin section cut from calcite crystals grown in agarose gels containing GO; (d) A SAED pattern of the thin section; (e-f) Raman intensity maps of an etched calcite crystal where calcite is identified (1050-1150 cm-1) in (e) and the distribution of GO is marked (1300-1400 cm-1) in (f). | |
In summary, we have demonstrated that the mechanical properties of gel-grown calcite single-crystal composites can be modified by incorporation of guest materials including agarose gel fiber, MWCNTs and GO. The incorporation of the guest materials has been evidenced by microscopy (OM, SEM, STEM) and Raman spectroscopy. For gel fibers and MWCNTs, they are uniformly incorporated and the TEM-SPM test shows that the obtained singlecrystalline composites exhibit greatly enhanced damage tolerance. The in situ nanoscale imaging reveals that the improved toughness is associated with the incorporated guest materials that shield the fracture by crack bridging. In contrast, the GO is not uniformly incorporated and the mechanical enhancement is not remarkable. Thisworkmay presentguidance on the rationaldesign and synthesis of mechanically strong single-crystal composites.
AcknowledgmentsThis work was supported by the 973 Program (No. 2014CB643503) andthe National Natural Science Foundation of China (Nos. 51625304, 51461165301). This research used resources of the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility, at Brookhaven National Laboratory under Contract No. DESC0012704. Y.Liu acknowledges financial support from the China Scholar Council.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.cclet.2018.05.044.
| [1] |
(a) P.M. Dove, J.J. DeYoreo, S. Weiner, Biomineralization, The Mineralogical Society of America, Washington DC, 2003, pp. 57-93; (b) Y. Politi, T. Arad, E. Klein, S. Weiner, L. Addadi, Science 306 (2004) 1161-1164; (c) J. Aizenberg, A. Tkachenko, S. Weiner, L. Addadi, G. Hendler, Nature 412 (2001) 819-822; (d) F. Nudelman, H.H. Chen, H.A. Goldberg, S. Weiner, L. Addadi, Faraday Dis. 136 (2007) 9-25; (e) A. Berman, J. Hanson, L. Leiserowitz, et al., Science 259 (1993) 776-779; (f) K. Gries, R. Kroger, C. Kubel, M. Fritz, A. Rosenauer, Acta Biomater. 5 (2009) 3038-3044. |
| [2] |
J. Aizenberg, J. Hanson, T.F. Koetzle, S. Weiner, L. Addadi, J. Am. Chem. Soc. 119 (1997) 881-886. DOI:10.1021/ja9628821 |
| [3] |
(a) H.Y. Li, H.L. Xin, M.E. Kunitake, et al., Adv. Funct. Mater. 21 (2011) 2028-2034; (b) J.S. Robach, S.R. Stock, A. Veis, J. Struct. Biol. 151 (2005) 18-29. |
| [4] |
L. Addadi, S. Weiner, Angew. Chem. Int. Ed. 31 (1992) 153-169. DOI:10.1002/anie.199201531 |
| [5] |
(a) B. Pokroy, A.N. Fitch, P.L. Lee, et al., J. Struct. Biol. 153 (2006) 145-150; (b) B. Pokroy, A.N. Fitch, E. Zolotoyabko, Adv. Mater. 18 (2006) 2363-2368. |
| [6] |
(a) M.E. Kunitake, S.P. Baker, L.A. Estroff, MRS Commun. 2 (2012) 113-116; (b) M.E. Kunitake, L.M. Mangano, J.M. Peloquin, S.P. Baker, L.A. Estroff, Acta Biomater. 9 (2013) 5353-5359. |
| [7] |
(a) R. Gueta, A. Natan, L. Addadi, et al., Angew. Chem. Int. Ed. 46 (2007) 291-294; (b) P. Gilbert, A. Young, S.N. Coppersmith, Proc. Natl. Acad. Sci. U. S. A. 108 (2011) 11350-11355; (c) P. Gilbert, R.A. Metzler, D. Zhou, et al., J. Am. Chem. Soc. 130 (2008) 17519-17527. |
| [8] |
(a) J.M. Garcia-Ruiz, J.A. Gavira, F. Otalora, A. Guasch, M. Coll, Mater. Res. Bull. 33 (1998) 1593-1598; (b) J.A. Gavira, A.E. Van Driessche, J.M. Garcia-Ruiz, Cryst. Growth Des. 13 (2013) 2522-2529; (c) Y.Y. Kim, K. Ganesan, P.C. Yang, et al., Nat. Mater. 10 (2011) 890-896; (d) A. Brif, G. Ankonina, C. Drathen, B. Pokroy, Adv. Mater. 26 (2014) 477-481; (e) H.Y. Li, H.L. Xin, D.A. Muller, L.A. Estroff, Science 326 (2009) 1244-1247; (f) Y.J. Liu, T.W. Yuan, Y. Shi, et al., Angew. Chem. Int. Ed. 53 (2014) 4127-4131; (g) C.H. Lu, L.M. Qi, H.L. Cong, et al., Chem. Mater. 17 (2005) 5218-5224; (h) L. Chen, T. Ye, X. Jin, et al., CrystEngComm 17 (2015) 8113-8118; (i) W. Liu, Y.J. Liu, L. Chen, et al., Chin. Chem. Lett. 26 (2015) 504-508; (j) B. Wucher, W.B. Yue, A.N. Kulak, F.C. Meldrum, Chem. Mater.19 (2007) 1111-1119; (k) M. Sindoro, Y. Feng, S. Xing, et al., Angew. Chem. Int. Ed. 50 (2011) 9898-9902; (l) Y.Y. Kim, A.S. Schenk, D. Walsh, et al., Nanoscale 6 (2014) 852-859. |
| [9] |
(a) R. Munoz-Espi, A. Chandra, G. Wegner, Cryst. Growth Des. 7 (2007) 1584-1589; (b) Y.Y. Kim, L. Ribeiro, F. Maillot, et al., Adv. Mater. 22 (2010) 2082-2086; (c) G. Lu, S. Li, Z. Guo, et al., Nat. Chem. 4 (2012) 310-316; (d) T. Ye, X.Y. Jin, L. Chen, et al., Chin. Chem. Lett. 28 (2017) 857-862; (e) Y.J. Liu, H. Zang, L. Wang, et al., Chem. Mater. 28 (2016) 7537-7543; (f) A.N. Kulak, R. Grimes, Y.Y. Kim, et al., Chem. Mater. 28 (2016) 7528-7536; (g) E. Asenath-Smith, J.M. Noble, R. Hovden, et al., Chem. Mater. 29 (2017) 555-563. |
| [10] |
(a) L.A. Estroff, I. Cohen, Nat. Mater. 10 (2011) 810-811; (b) K. Rae Cho, Y.Y. Kim, P. Yang, et al., Nat. Commun. 7 (2016) 10187. |
| [11] |
(a) H.J. Nickl, H.K. Henisch, J. Electrochem. Soc. 116 (1969) 1258-1270; (b) J.A. Gavira, J.M. Garcia-Ruiz, Acta Crystallogr. Sect. D-Biol. Crystallogr. 58 (2002) 1653-1656; (c) H.Y. Li, L.A. Estroff, Adv. Mater. 21 (2009) 470-473; (d) J. Ren, B. Huang, L. Chen, et al., CrystEngComm 18 (2016) 800-806; (e) H. Li, G. Xue, J. Wu, et al., Chin. Chem. Lett. 28 (2017) 2121-2124. |
| [12] |
(a) S. Borukhin, L. Bloch, T. Radlauer, et al., Adv. Funct. Mater. 22 (2012) 4216-4224; (b) Y.Y. Kim, J.D. Carloni, B. Demarchi, et al., Nat. Mater. 15 (2016) 903-910; (c) B. Kahr, R.W. Gurney, Chem. Rev. 101 (2001) 893-951. |
| [13] |
(a) E. Asenath-Smith, H.Y. Li, E.C. Keene, Z.W. Seh, L.A. Estroff, Adv. Funct. Mater. 22 (2012) 2891-2914; (b) M.D. Giosia, I. Polishchuk, E. Weber, et al., Adv. Funct. Mater. 26 (2016) 5569-5575; (c) J.Chmielewski, J.J. Lewis, S. Lovell, etal., J. Am.Chem. Soc.119 (1997) 10565-10566; (d) J.B. Benedict, P.M. Wallace, P.J. Reid, S.H. Jang, B. Kahr, Adv. Mater.15 (2003) 1068-1070; (e) E.J.W. Crossland, N. Noel, V. Sivaram, et al., Nature 495 (2013) 215-219. |
| [14] |
C. Hu, T. Ye, Y.J. Liu, et al., Mater. Chem. Front. 2 (2018) 363-368. |
| [15] |
M. Calvaresi, G. Falini, L. Pasquini, et al., Nanoscale 5 (2013) 6944-6949. DOI:10.1039/c3nr01568h |
| [16] |
A.S. Schenk, I. Zlotnikov, B. Pokroy, et al., Adv. Funct. Mater. 22 (2012) 4668-4676. DOI:10.1002/adfm.v22.22 |
| [17] |
J.Y. Huang, H. Zheng, S.X. Mao, Q. Li, G.T. Wang, Nano Lett. 11 (2011) 1618-1622. DOI:10.1021/nl200002x |
| [18] |
H.Y. Li, L.A. Estroff, J. Am. Chem. Soc. 129 (2007) 5480-5483. DOI:10.1021/ja067901d |
| [19] |
(a) M.M.J. Treacy, T.W. Ebbesen, J.M. Gibson, Nature 381 (1996) 678-680; (b) J. You, J.Y.Q. Cao, S.C. Chen, Y.Z. Wang, Chin. Chem. Lett. 28 (2017) 201-205. |
| [20] |
(a) M.S. Dresselhaus, G. Dresselhaus, R. Saito, A. Jorio, Phys. Rep. 409 (2005) 47-99; (b) C.G. Kontoyannis, N.V. Vagenas, Analyst 125 (2000) 251-255. |
| [21] |
D.P. Schweinsberg, Y.D. West, Spectrochim. Acta A 53 (1997) 25-34. |
| [22] |
(a) Z. Zhang, M.M. Mao, J. Wang, et al., Nat. Commun. 6 (2015) 10143; (b) R.K. Nalla, J.J. Kruzic, J.H. Kinney, R.O. Ritchie, et al., Biomaterials 26 (2005) 217-231; (c) R.O. Ritchie, Mater. Sci. Eng. A 103 (1988) 15-28; (d) R.O. Ritchie, Int. J. Fracture 100 (1999) 55-83; (e) A.G. Evans, J. Am. Ceram. Soc. 73 (1990) 187-206. |
| [23] |
(a) M.H.G. Wichmann, K. Schulte, H.D.Wagner, Compos. Sci.Technol. 68 (2008) 329-331; (b) H.D. Wagner, P.M. Ajayan, K. Schulte, Compos. Sci. Technol. 83 (2013) 27-31. |
| [24] |
D.R. Dreyer, S. Park, C.W. Bielawski, R.S. Ruoff, Chem. Soc. Rev. 39 (2010) 228-240. DOI:10.1039/B917103G |
| [25] |
X. Wang, H. Bai, Y. Jia, et al., RSC Adv. 2 (2012) 2154-2160. DOI:10.1039/c2ra00765g |
| [26] |
A.E. Nielsen, Pure Appl. Chem. 53 (1981) 2025-2039. DOI:10.1351/pac198153112025 |
| [27] |
D. Yang, A. Velamakanni, G. Bozoklu, et al., Carbon 47 (2009) 145-152. DOI:10.1016/j.carbon.2008.09.045 |
| [28] |
I. Chowdhury, M.C. Duch, N.D. Mansukhani, et al., Environ. Sci. Technol. 47 (2013) 6288-6296. DOI:10.1021/es400483k |
 2018, Vol. 29
2018, Vol. 29 


