Supramolecular polymers [1] refer to those structures constructed from monomers by non-covalent interactions including hydrogen bonding, aromatic stacking, host-guest interaction, and metal coordination etc. [2-4]. Owing to their dynamic properties such as self-healing, reversibility or stimuli-responsiveness [5], supramolecular polymers and materials have attracted increasing interests from chemists all over the world. Among the stimuli such as light, temperature, pH, metal ions and solvent composition [6], light represents an outstanding position because of its non-contact approach and as a cleaning sustainable energy resource [7]. Many reversible, weak noncovalent interactions have been introduced to fabricate the directional polymeric arrays by repeating monomer units. Among the noncovalent interactions, host-guest interaction, which endows the supramolecular polymers many interesting and fascinating chemical and physical properties [8], plays an important role in the process of supramolecular polymerization because of their fast self-selectivity and efficient self-assembly property. During the past decades, as a basic building block, crown ether [9] has been widely used to construct host-guest interaction based supramolecular polymers. Huang and coworkers have developed some supramolecular polymer systems with different crown ether hosts and complicated guest molecules [10].
In general, supramolecular polymer, extended from supramolecular chemistry and polymer science, have the easy and efficient construction advantages [11] while the drawbacks of difficulty in precise control and morphologic adjustment which is special important for the construction of novel smart materials [12]. Because of the unique property of light stimuli, various photo-responsive chromophores were introduced to the supramolecular monomer to fabricate the photo-responsive assemblies [13]. Recently we reported the photo-triggered supramolecular polymerization of a [c2] daisy chain rotaxane which was decorated by two terminal photocleavable monomeric units that can form supramolecular polymer through quadruple hydrogen bonding under irradiation of UV light [14].
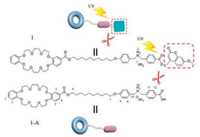
Inspired by the combination of polymerization induced by photo-irradiation and host-guest self-assembly, here we designed and synthesized a novel low molecular weight AB-type heteroditopic monomeric building block as the repeating units to form supramolecular polymer. As shown in Scheme 1, compound 1 contains one crown ether moiety (DB24C8) as a macrocyclic ring on one side and one dibenzylammonium salt (DBA) as a recognition site. The other side of monomer was decorated with a coumarin group as a photocleavable protector which is large enough for cavity of the host to prevent monomer forming supramolecular polymer. Irradiation of 1 with 320 nm UV light can result in the cleavage of the coumarin unit [15] and the formation of compound 1-A, which has no photocleavable protector and can self-assemble into oligomer or supramolecular polymer at different concentrations spontaneously because of the host-guest interaction between the DB24C8 and DBA units.
|
Download:
|
| Scheme 1. Schematic representation of the formation of the compounds 1 and 1-A. | |
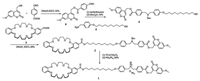
The preparation of compound 1 was shown in Scheme 2. The esterification reaction between the 7-methoxy coumarin and 4-carboxybenzaldehyde under the condition of 4-dimethylamiopryidine (DMAP) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) in dry dichloromethane resulted in the formation of compound 6 in a 95% yield. Then, the Schiff base reaction between compound 6 and 5 was carried out in toluene and refluxed for 10 hours. The subsequent reduction reaction with NaBH4 in methyl alcohol and then protected by di-tert-butyl pyrocarbonate can obtain compound 4 in a yield of 56%. Next, the important intermediate 2 was obtained byan esterification reaction between compound 3 and 4. Finally, monomer 1 was obtained by treatment of compound 2 with trifluoroacetic acid, followed by ion exchange with NH4PF6 aqueous solution. The synthesis of compound 1-A was generally the same with compound 1 (the detailed synthesis route was shown in Supporting information).
|
Download:
|
| Scheme 2. The chemical structure and preparation of compound 1. | |
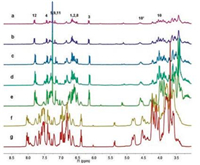
The structure of compound 1 was confirmed by 1H NMR spectroscopy and mass spectrometry. The proton resonances of compound 1 in CDCl3 were well distributed and mutually independent, revealing the monodispersed state of monomer 1 and indicating the coumarin group was large enough for the cavity of macrocyclic host to form host-guest interaction-based supramolecular polymers (Figures were shown in Supporting information). Then the properties of model compound 1-A were examined. As shown in Fig. 1, the concentration-dependent 1H NMR spectra of compound 1-A at varying concentrations from 2.0 mmol/L to 100.0 mmol/L were obtained, providing an important view on the self-assembly process of the supramolecular oligomer and polymer (CD3CN:CDCl3 = 1:1, v/v). The 1H NMR was complicated because of the slow exchange complexation of the DB24C8 and DBA units on the proton NMR time scale. Notably, at a lower concentration, the 1H NMR spectrum of compound 1-A was more complicated than that of compound 1, suggesting the formation of low molecular weight oligomers. With increasing concentrations of monomer 1-A, obvious broadening and gathering of proton signals could be observed, indicating the formation of high molecular weight supramolecular polymers.
|
Download:
|
| Fig. 1. Partial 1H NMR spectra (400 MHz, CD3CN/CDCl3 = 1:1, 298 K) of equivalent molar solutions of compound 1-A at different concentrations (a) 2.0 mmol/L; (b) 6.0 mmol/L; (c) 10.0 mmol/L; (d) 20.0 mmol/L; (e) 40.0 mmol/L; (f) 60.0 mmol/L and (g) 100.0 mmol/L. | |
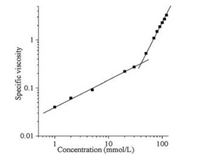
Double logarithmic plot of specific viscosity versus the concentration is always an easy and reliable method to investigate the propensity of monomers self-assemble into high molecular weight polymers. To further investigate the supramolecular polymerization process of compound 1, the specific viscosity of compound 1-A in different concentration was studied in equimolar dichloromethane solutions. As shown in Fig. 2, when at a lower concentration, the viscosity of the compound 1-A solution increased slowly and the slope of curve was 0.56, consistent with the concentration of monomers or cyclic oligomers. With the concentration increased, it can be observed that the slope of curve suddenly increased from 0.56 to 2.09, meaning the existence of high molecular weight supramolecular polymers. In addition, the change of the slope also revealed that the supramolecular polymerization was concentration-dependent. The critical polymerization concentration (CPC) for monomer 1-A was about 40 mmol/L as evidenced by the clear change of the slope happening at this concentration, suggesting the low molecular weight oligomers self-assembling into supramolecular polymers due to the host-guest interaction between the DB24C8 ring and DBA recognition site.
|
Download:
|
| Fig. 2. Log–log plot of specific viscosity of equimolar solutions of 1-A versus the monomer concentration at 298 K. | |
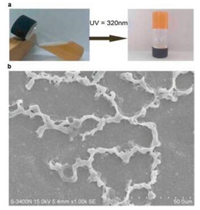
Then, the characteristic of compound 1 was studied based on the research of compound 1-A. The photocleavable protector containing AB-type heteroditopic monomer was exposed to daylight for one day and no obvious changes were found in solution state. When ultraviolet light (λ = 320 nm) was used to irradiate compound 1, the solution converted to supramolecular organic gel after 6 h (Fig. 3a). It can be attributed to the fact that the coumarin group was large enough to prevent the DBA recognition site get inside the DB24C8 macrocycle in the beginning. After irradiated with UV light, the big coumarin blocking stopper underwent photocleavage so that made the guest motif free and threaded into the DB24C8 macrocycle of another monomer. When the solution was concentrated enough, supramolecular polymer gel would form eventually. The morphology of supramolecular gel examined by scanning electron microscopy, proving the monomer 1 aggregated into dimensional fibers (Fig. 3b).
|
Download:
|
| Fig. 3. (a) The gel–sol transitions of the supramolecular network triggered by UV light (λ = 320 nm); (b) SEM images of the supramolecular polymer gel. | |
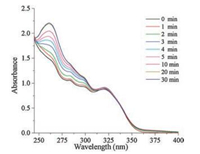
To further study the photocleavage process, the absorption spectra of the compound 1 solution (CHCl3:CH3CN: H2O = 100:100:1, v/v/v) was investigated after incremental minutes of irradiation by UV light. As shown in Fig. 4, compound 1 exhibited two absorption bands with λmax at 260 nm and 320 nm, respectively. Increasing the irradiation time, there exist increase on the peak at 260 nm and decrease at λ = 320 nm gradually. The peak appeared at 260 nm can be attributed to the increasing concentration of compound 1-A solution. To make more detailed evaluation, the absorption spectra of the compound 1-A in the same solution was carried out (Fig. S22 in Supporting information), showing that there was also a strong absorption peak centered at 260 nm. Besides, the HR-ESI mass spectrometry under the irradiation by UV-light also revealed the compound 1-A was released and the photolysis was happened.
|
Download:
|
| Fig. 4. The absorption spectra of compound 1 irradiated by UV-light after incremental minutes. | |
In conclusion, a new type of host-guest interaction-based supramolecular polymer gel combined both the process of photoinduced polymerization and host-guest interaction was prepared and investigated. Under the irradiation with UV light (λ = 320 nm), the photocleavage process occurred and make the DBA unit released and combined with DB24C8, thus a light controllable supramolecular polymerization was achieved. We envisage such a new photo fabricating method would enrich the supramolecular polymer family and this method would provide a controllable and efficient way to construct supramolecular materials. Moreover, photo-induced supramolecular polymer and nanostructures are extremely important in the areas of bioscience, medical science and cell biology. The research on more photocleavable protectors by two-photo process is still going on.
AcknowledgmentsWe thank the support of the National Natural Science Foundation of China (No. 21672060), the Fundamental Research Funds for the Central Universities (Nos. WJ1616011, WJ1213007, 222201717003), the Programme of Introducing Talents of Discipline to Universities (No. B16017).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.04.002.
| [1] |
(a) L. Brunsveld, B.J.B. Folmer, E.W. Meijer, R.P. Sijbesma, Chem. Rev.101 (2001) 4071-4097; (b) T.F.A. De Greef, M.M.J. Smulders, M. Wolffs, et al., Chem. Rev. 109 (2009) 5687-5754; (c) W. Tian, X. Li, J. Wang, Chem. Commun. 53 (2017) 2531-2542. |
| [2] |
(a) Z. Qi, C.A. Schalley, Acc. Chem. Res. 47 (2014) 2222-2233; (b) A. Harada, Y. Takashima, H. Yamaguchi, Chem. Soc. Rev. 38 (2009) 875-882; (c) S.L. Li, T.X. Xiao, C. Lin, L.Y. Wang, Chem. Soc. Rev. 41 (2012) 5950-5968; (d) H. Zhang, J. Hu, D. Qu, Org. Lett. 14 (2012) 2334-2337; (e) W. Wang, C. Gao, Q. Zhang, X. Ye, D. Qu, Chem. Asian J. 12 (2017) 410-414. |
| [3] |
C. Xu, L. Xu, X. Ma, Chin. Chem. Lett. 29 (2018) 970-972. DOI:10.1016/j.cclet.2017.11.045 |
| [4] |
Q. Wang, M. Cheng, J. Jiang, L. Wang, Chin. Chem. Lett. 28 (2017) 793-797. DOI:10.1016/j.cclet.2017.02.008 |
| [5] |
(a) B. Zheng, F. Wang, S. Dong, F. Huang, Chem. Soc. Rev. 41 (2012) 1621-1636; (b) T. Aida, E.W. Meijer, S.I. Stupp, Science 335 (2012) 813-817; (c) L. Yang, X. Tan, Z. Wang, X. Zhang, Chem. Rev. 115 (2015) 7196-7239; (d) Z. Li, Y. Zhang, C. Zhang, et al., J. Am. Chem. Soc. 136 (2014) 8577-8589; (e) H. Li, X. Fan, W. Tian, et al., Chem. Commun. 50 (2014) 14666-14669. |
| [6] |
(a) D. Qu, Q. Wang, Q. Zhang, X. Ma, H. Tian, Chem. Rev.115 (2015) 7543-7588; (b) P. Kuad, A. Miyawaki, Y. Takashima, H. Yamaguchi, A. Harada, J. Am. Chem. Soc. 129 (2007) 12630-12631; (c) J. Xu, Y. Chen, L. Wu, C.H. Tung, Q. Yang, Org. Lett. 15 (2013) 6148-6151; (d) S. Amharar, S. Yuvayapan, A. Aydogan, Chem. Commun. 54 (2018) 829-832; (e) W. Wang, Q. Gao, A. Li, et al., Chin. Chem. Lett. 29 (2018) 336-338. |
| [7] |
(a) M. Ouchi, N. Badi, J.F. Lutz, M. Sawamoto, Nat. Chem. 3 (2011) 917-924; (b) M. Ikeda, T. Tanida, T. Yoshii, I. Hamachi, Adv. Mater. 23 (2011) 2819-2822; (c) T. Mes, R. van der Weegen, A.R.A. Palmans, E.W. Meijer, Angew. Chem. Int. Ed. 50 (2011) 5085-5089; (d)N. Hosono, M.A.J. Gillissen, Y. Li, et al., J. Am.Chem. Soc.135 (2012) 501-510; (e) P.J.M. Stals, Y. Li, J. Burdynska, et al., J. Am. Chem. Soc. 135 (2013) 11421-11424; (f) J. Gui, Z. Yan, Y. Peng, et al., Chin. Chem. Lett. 27 (2016) 1017-1021; (g) W. Wua, S. Song, X. Cui, et al., Chin. Chem. Lett. 29 (2018) 95-98. |
| [8] |
(a) X. Yan, S. Li, J.B. Pollock, et al., Proc. Natl. Acad. Sci. U. S. A.110 (2013) 15585-15590; (b)J.F. Xu, Y.Z. Chen, D.Y. Wu, et al., Angew. Chem. Int. Ed. 52 (2013) 9738-9742; (c) H.Q. Peng, J.F. Xu, Y.Z. Chen, et al., Chem. Commun. 50 (2014) 1334-1337; (d) F. Wang, C. Han, C. He, et al., J. Am. Chem. Soc. 130 (2008) 11254-11255; (e) Z. Zhang, Y. Luo, J. Chen, et al., Angew. Chem. Int. Ed. 50 (2011) 1397-1401. |
| [9] |
(a) J.W. Jones, W.S. Bryant, A.W. Bosman, et al., J. Org. Chem. 68 (2003) 2385-2389; (b) H. Li, X. Fan, X. Shang, et al., Polym. Chem. 7 (2016) 4322-4325. |
| [10] |
S. Dong, L. Gao, J. Li, D. Xu, Q. Zhou, Polym. Chem. 4 (2013) 3968-3973. DOI:10.1039/c3py00494e |
| [11] |
S. Dong, Y. Luo, X. Yan, et al., Angew. Chem. Int. Ed. 50 (2011) 1905-1909. DOI:10.1002/anie.v50.8 |
| [12] |
X. Yan, F. Wang, B. Zheng, F. Huang, Chem. Soc. Rev. 41 (2012) 5869-6216. DOI:10.1039/c2cs90072f |
| [13] |
(a) T. Muraoka, K. Kinbara, Y. Kobayashi, T. Aida, J. Am. Chem. Soc. 125 (2003) 5612-5613; (b) P. Mobian, J.M. Kern, J.P. Sauvage, Angew. Chem. Int. Ed. 43 (2004) 2392-2395; (c) J. Berná, D.A. Leigh, M. Lubomska, et al., Nat. Mater. 4 (2005) 704-710; (d) T. Muraoka, K. Kinbara, T. Aida, Nature 440 (2006) 512-515; (e) A. Credi, M. Venturi, V. Balzani, ChemPhysChem 11 (2010) 3398-3403; (f) X. Ma, H. Tian, Chem. Soc. Rev. 39 (2010) 70-80. |
| [14] |
(a) X. Fu, R. Gu, Q. Zhang, et al., Polym. Chem. 7 (2016) 2166-2170; (b) G. Antoine, M. Giacomo, L. Thomas, et al., J. Am. Chem. Soc. 139 (2017) 4923-4928. |
| [15] |
R. Schmidt, D. Geissler, V. Hagen, J. Bendig, J. Phys. Chem. A 116 (2007) 5768-5774. |
 2018, Vol. 29
2018, Vol. 29 


