b International Laboratory for Insulation and Energy Efficiency Materials, College of Materials Science and Technology, Nanjing University of Aeronautics and Astronautics, Nanjing 210016, China;
c Shandong Huaying New Material Co., Ltd., Heze 274000, China;
d School of Mechanical Engineering, Changzhou University, Changzhou 213164, China;
e Department of Obstetrics and Gynecology, Zhujiang Hospital, Southern Medical University, Guangzhou 510282, China;
f Guangdong Provincial Key Laboratory of New Drug Screening, School of Pharmaceutical Sciences, Southern Medical University, Guangzhou 510515, China
Chemotherapy is an important strategy for malignant tumors treatments, in which small molecule drugs take up the main part as the clinically chemotherapeutic drugs [1-3]. The development of tumor-targeted nanomaterials for small molecule drugs delivery cannot only achieve the targeted delivery, but also make the small molecule drugs control-release in the tumor site, which significantly reduce the toxicity and side effects of drugs [4-7]. During the last decades, scientists have devoted great efforts to develop the targeted anti-cancer drug delivery systems, including magnetic nanoparticles [8-10], polymeric nanoparticles [11-17], mesoporous silica nanoparticles [18, 19], liposomes [20-22], peptide [23-26], and small molecules [27-29]. These drug carriers have shown promising applications for the delivery of small molecule drugs, proteins and nucleic acids [30-33]. Within the nanoparticle family, magnetic nanoparticles exhibit promising potential as the delivery systems for a wide range of diseases treatments, due to the structural flexibility, which can be modified with intricate definition over their compositions, structures and properties.
Magnetic nanoparticles (MNPs) have been researched widely for their magnetic properties and prospective applications [34-36]. Since first reported in 2000, MNPs were extensively used in the field of nanotechnology for its nonhazardous feature [37], strong magnetization values [38], superparamagnetic property [39, 40], active surface that can easily assembled of biological soluble structure [41] and targeting, imaging, and therapeutic molecules [42, 43]. Advances in technology accelerated the evolution of MNPs that overcome the faults of previous cancer diagnosis and treatment materials [44]. The characteristic of MNPs can be easy to be multi-functional modified and rapidly response to the magnetic environment has made them an ideal material for tissue repair [45-47], biosensor [48, 49], immunoassay, detoxification of biological fluids, data storage [50-52], environmental remediation [53], hyperthermia [54], and cell separation [55]. By multi-functional processing, MNPs have function of both magnetic resonance imaging (MRI) [56-58] and medicine carriers [59, 60]. MNPs-based MRI has been widely used in cell replacement [61], surgical cutting and other aspects [62-65]. Although a number of preparation ways of MNPs have been successfully prepared, the use of MNPs is mainly up to the stability of the materials in different environments. Studies found that MNPs would have a more excellent performance when the size was less than 10–20 nm [66]. Over the years, significant progress has been made to precisely control the size of MNPs [67]. The synthesis has even led to the development of more exotic MNPs with control over their shapes, compositions, structures, and multifunctionality. Such controls achieved in MNPs size monodispersity have enabled more fundamental understandings of MNPs properties for nanomedicine applications [68-71].
However, an unavoidable trouble with the applications of MNPs is that their stability will decrease after a long using time, and material types, morphology and size can affect their instability [72]. Due to the large surface-to-volume ratio, MNPs easily assemble. Besides, metallic particles are chemically active and easily oxidized in an airy environment, which causes them to lose their magnetic property and solubility. Therefore, it is important to study the stability technology of MNPs in many applications. These techniques include the addition of an organic protective layer or an inorganic protective layer. It is gratifying that, in a lot of applications, these protective layers not only have the role of improving stability, but also have a number of special function.
Functionalized MNPs is well suited for applications in immunoassay, biomarkers and cell separation. In particular, in the liquid phase catalytic reaction, MNPs can be used as a uniform system with ideal dispersibility, large reactivity and excellent dispersibility. This article will summarize the current MNPs preparation, functionalization, and stabilization methods. It also summarizes the detailed features of MNPs. At last, detailed descriptions of the use in the areas of disease therapy, drug delivery, hyperthermia, vitro bioseparation and bioimaging will be presented.
2. Special features of MNPsCharacteristics of MNPs are different from the general magnetic materials and magnetic properties associated with the characteristics of the physical length of just for the nanoscale, and the electron mean free path, etc., generally in the 1~100 nm orders of magnitude, or magnetic body size and characteristics physical length is quite showing the anomalous magnetic and electrical properties. Different classification of MNPs differs materially from those features. Magnetic nanoparticles exhibit many important features, such as high specific surface area, chemical stability, low intraparticle, diffusion rate, high loading capacity, superparamagnetism. Most notably, superparamagnetism when the size is below certain critical dimensions [73, 74]. The critical diameter is material dependent like 35 nm for Fe3O4 [75]. Superparamagnetic nanoparticles provide a strong resistance to an outside field and exhibit very large saturation magnetization values, many orders of magnitude higher than what is observed in paramagnetic materials [76]. Moreover, their magnetic moment vectors relax to random directions Under the lack of applied magnetic field in the case, have no attraction for each other, thereby reducing the risk of particle's accumulation [77, 78].
2.1. Quantum size effectMaterial level spacing and atomic number N is proportional inversely. The atomic number is limited in the particles when the particle size is low to a certain degree. The electronic energy levels of nano metal near to the fermi level are from the continuous into discrete. There are the highest and lowest discontinuous occupied molecular orbital in nanometer semiconductor particle energy gap widened. When the energy gap spacing is bigger than the material properties of thermal energy, magnetic energy, electrostatic energy, photon energy and so on, will lead to nanoparticle characteristics and macroscopic physical properties of the materials were significantly different. Conductive metal, for example, in the superfine particles can become an insulator, magnetic moment and the size of the particles in the electron is odd or even number is related to abnormal heat will also change, spectral line will move in the direction of short wavelength, that is the macroscopic quantum size effect.
2.2. Surface effectsDue to the composition and nano particle size of nano materials, particle surface possession of the atomic number of the same quality than the possession of the atomic number material nanoparticles surface. With the decrease of particle size, the surface of geometric of particle number increases. Unit mass with the increase of particle surface area and a surge in surface atomic number, make a serious shortage of atomic coordination number, high surface area of the high surface energy at the same time, make the particle surface atoms is extremely active, easily with the surrounding gas, also easily adsorbed gas. This phenomenon is called the surface effect of nanometer materials. Use of this nature, people can use of nanomaterials in many ways to improve the utilization rate of material and develop new USES of nanomaterials [79]. The surface spins make a great contribution to the magnetism of the nanoparticles in the case of the large ratio of surface atoms/bulk. Visibly, the surface effects affect the performance of the magnetic nanoparticles.
2.3. Small size effectOwing to the size of the magnetic nanoparticles small enough to be with the magnetic exchange of length, wavelength, width of magnetic domain wall, conduction electrons DE Broglie wavelength, superconducting state coherence length or more physical characteristic length, the original crystal periodic boundary conditions are destroyed, physical properties also show that the new effects, such as from magnetic ordered into magnetic disorder, magnetic coercive force's change, the melting point's drop, etc.
3. Synthesis of MNPsUntil now, people have done a lot of researches on synthesis of magnetic nanoparticles. There are some popular ways to achieve shape-controllable, monodisperse, and excellently stable MNPs, such as sol-gel synthesis, co-precipitation, hydrothermal synthesis, thermal decomposition, microemulsion, sonolysis and biosynthesis. To date, MNPs prepared from co-precipitation and thermal decomposition are the best studied, and they can be prepared on a large scale.
3.1. Co-precipitationCo-precipitation technology may be the most convenient and effective ways to obtain magnetic particles. Generally, the aqueous Fe2+/Fe3+ salts solution is the reactant of iron oxides. As shown in Fig. 1, the Fe2+/Fe3+ ratio, pH value, temperature, and nature of the solvent can have important effects on the composition and dimension of the nanoparticles [80, 81]. For example, to obtain pure Fe3O4, pH value have to be controlled from 8 to 14 and Fe2+/Fe3+ ratio should be 1:2 [82].

|
Download:
|
| Fig. 1. TEM images of MNPs. (a) 8 nm/2.5 nm Fe/Fe3O4 NPs (inset: HRTEM). (b) 5 nm/5 nm Fe/Fe3O NPs prepared by controlled oxidation of Fe NP. Reproduced with permission [80]. Copyright 2006, American Chemical Society). (c) Fe3O4 particles obtained by co-precipitation in solution. Reproduced with permission [81]. Copyright 2007, Elsevier. | |
3.2. Thermal decomposition
In terms of size and morphology control of the nanoparticles, thermal decomposition seems the best method developed to date although it is complicated. As shown in Fig. 2, the organometallic compounds dissolved in nonpolar solvent can be used for preparing monodisperse pony-size nanocrystals by the thermal decomposition under the help of stabilizing surfactants [83-85]. Metal cupferronates, metal acetylacetonates and carbonyls are often used as the organometallic precursors [86]. Surfactants usually include fatty acids, oleic acid, as well as hexadecylamine [87]. In principle, the shape and size of nanoparticles are largely depended on the proportion of organometallic precursors, surfactants, and solvents. In addition, reaction time, reaction temperature, as well as aging period may also be important factors.

|
Download:
|
| Fig. 2. Metal-oleate precursors were prepared from the reaction of metal chlorides and sodium oleate. (a) The thermal decomposition of the metal-oleate precursors in the high boiling solvent produced monodisperse nanocrystals. Reproduced with permission [83]. Copyright 2004, Nature Publishing Group. (b)Transmission electron microscopy (TEM) images of 6, 7, 8, 9, 10, 11, 12, and 13 nm-sized airoxidized IONPs showing the one nanometer level increments in diameter. Reproduced with permission [84]. Copyright 2005, John Wiley and Sons. | |
3.3. Hydrothermal syntheses
The hydrothermal synthesis is usually applied in aqueous media in reaction kettles at high pressure and high temperature to produce magnetic nanoparticles [88]. High temperature is beneficial to improve the nucleation rate and speed up the growth of the new particles, which results in the small size of particles. Hydrolysis and oxidation is one of the main routes for obtaining nanoparticles under hydrothermal conditions, and another route is neutralization of hybrid metal hydroxides. By the means of controlling the technological parameters, including the temperature, the reaction time, the concentration of reactants, and the characteristics of the solvent, the geometric parameter of the nanoparticles can be optimized. This method is a relatively little explored method for the synthesis of magnetic nanoparticles, although it allows the synthesis of high-quality nanoparticles.
3.4. Sol–gel synthesisFor the sol-gel synthesis, the hydroxylation and condensation of reactants in solutions are used for achieving a sol of nanoparticles. Then a gel with a three-dimensional network of metal oxide is created through the condensation as well as inorganic polymers of aerosol. By the heat treatment of the gel, the crystalline state can be gained [89]. By the means of optimizing the condensation and hydroxylation conditions, especially the temperature, the pH value, the nature of the solvent, the property and concentration of the salt precursors, the structure and performance of the gel can be controlled easily [90]. And by adding surfactants into the solution phase, the sol-gel synthesis can be optimized with ease. This method has many kinds of reactants, good mixing uniformity, high reaction activity, low synthesis temperature and the synthesis process is easy to control. However the material will inevitably be coagulated during post-treatment.
3.5. MicroemulsionThe microemulsion is a kind of liquid mixture of water, oil and surfactant, with the nature of stability and isotropy [91]. In microemulsions, the hydrocarbon phase is continuous and the aqueous phase is dispersed as microsphere, which is surrounded by the singer-layer surfactant molecules. The size of the reverse micelle depends on the molar ratio of surfactant to water [92]. After blending two same microemulsions with relevant chemicals, there will be some reactions come up of the microdroplets including collide, coalesce, and break again, and the sediment will occur in the end [93]. For the purpose of extracting the precipitate, acetone or ethanol or other organic solvent can be add to the mixture and then filtration or centrifugation can be carried out. From the above, the nanoparticles can be yield by the microemulsion, which plays a role of nano creator.
3.6. SonolysisFor the sonolysis method, new structures can be produced through high intensity ultrasound, and it has no need for high pressure, high temperature or long time for reaction, which make it be a unusual route. Under ultrasonic radiation, some cavities can be achieved by the commutative expansive and compressive acoustic waves and the cavities are oscillating. When the oscillating cavities grow to a certain size, the ultrasonic energy can be accumulated by them. After that, the energy reserved in the cavities can be freed in a moment after the cavities overgrow and collapse suddenly [94, 95]. As a result, the sonolysis method is usually applied to achieve different types of bare and functionalized nanoparticles.
3.7. Biological synthesisIn principle, the biosynthesis method is an environment friendly route and the resultants have a fine biocompatibility. The plant phytochemicals and the microbial enzymes, which have reducing properties and antioxidant, are often used to reduce the salts into nanoparticles. For instance, iron reducing bacteria and magnetotactic bacteria can be applied to the biosynthesis of iron oxide nanoparticles [96, 97]. However, the exact mechanism of biological synthesis has not been explained clearly, and the shape and size of the nanoparticles can't be controlled precisely.
4. Functionalization of MNPsTo provide magnetic nanoparticles with the stabilization in nonaqueous solvents, the nanoparticles are usually wrapped with a hydrocarbon layer. In order to apply the nanoparticles in biomedicine, the surface functionalization must be carried out for these nanoparticles to get hydrophilic and biocompatible properties [98]. Ligand addition, ligand exchange and hydrophilic silica coating are the three most important methods for the surface functionalization.
4.1. Ligand additionThe molecules containing both a hydrophilic group and a hydrophobic group, as called amphiphilic molecules, are employed to the ligand addition [99, 100]. In principle, a double layer structure around the nanoparticles can be formed by the hydrophobic groups and the initial hydrocarbon layer. And the hydrophilic segments are outermost of the nanoparticles, which make them be soluble in water.
For the surface modification of high moment SPM FeCo particles, Dai's team has invented a phospholipid micelle coating modified with PEG [101]. The graphitic shells of FeCo particles are connected with the hydrocarbon chains of the phospholipid to achieve steady double layers, while the PEG chains are located in the external position of the double layers to make them watersoluble. Besides, Shin et al. adopted similar method to offer the manganese oxide nanoparticles with admirable biocompatibility through the PEG-phospholipid shell [102].
4.2. Ligand exchangeFor ligand exchange, a new type of coordinating group is applied to replace the initial hydrocarbon layer directly. The new ligand has two different kinds of functional groups, including a functional group which can link to the surface of the nanoparticles tightly by the effect of chemical boding and another functional group with polarity at the other side to make the particles dissolved in water solution.
Rotello and his colleagues have found that in the effect of octa polyhedral oligomeric silsesquioxane, the iron oxide nanoparticles dissolved in toluene solution can become soluble in water [103]. What makes it meaningful, many kinds of magnetic nanoparticles with different monolayer can be functionalized by the TMA-POSS, such as iron oxide nanoparticles with oleic acid, and Fe/Pt nanoparticles with oleylamine, oleic acid and hexadecanediol.
4.3. Silica coatingSilica coating is widely applied to the surface functionalization of nanoparticles to improve their stability in water and biological compatibility [104, 105]. The sol-gel process is the most common approach to obtain silica shell, including the hydrolysis of tetraethyl orthosilicate (TEOS) and succedent condensation [106, 107]. The reaction duration, the amount of nanoparticles and the concentration of TEOS are key factors to control the thickness of the coating. Before the route starting, the nanoparticles in nonpolar solvent have to be transferred to water phase, with the help of hydrophilic ligands like PVP and CTAB [108, 109].
Xia and his colleagues have published that the hydrolysis of TEOS can be used for the silica coating of the ferrofluids available in commercial [107]. Kobayashi and co-workers have developed a new approach, where TEOS and 3-aminopropyl trimethoxysilane are used as the precursors to gain the nanoparticles of Co with silica coating [110]. Shi et al have described the route of producing magnetic nanopheres with a mesoporous silica coating, which involve the Stöber process and sol-gel polymerization [111]. The comparison of advantages and disadvantages of different functionalization methods is summarized in Table 1.
|
|
Table 1 The comparison of different functionalization methods. |
5. Stabilization of MNPs
To stabilize these magnetic nanoparticles in the application without aggregation or precipitation for a long time, it is necessary to make the chemical stability of magnetic nanoparticles with efficient strategies better. Stabilization is critical for magnetic nanoparticles in almost any application fields. They are extremely reactive toward oxidation in the water or humid air. The equilibrium between repulsive and attractive forces results in the steadiness of a magnetic colloidal suspension. In the opinion of force analysis, in the system three types of forces are related to the interparticle potential. The repulsive force contain electrostatic and steric repulsion [112]. Adding salt to the suspension, partially shield the electrostatic repulsive forces. The non-naked particles must be considered in the steric repulsion forces [113]. Intermolecular forces contain strong short-range isotropic attractions [114]. Finally, magnetic dipolar forces between two particles have to be taken into account for magnetic suspensions. The most straightforward method seems to use appropriate surface coating, so that surface of the magnetic particles cannot reach the oxygen. As shown in Fig. 3, the alternative coating ways are separated into two major parts: organic coating includes polymers and surfactant [115-117], or inorganic components coating [118] includes carbon, silica, precious metals or oxides, such as Y2O3. It is a shell coats that the naked magnetic nanoparticle become a core, isolating the core against the air. Obviously, individually nanocrystals with coating are allodially dispersible and steady kinds of media [119, 120].
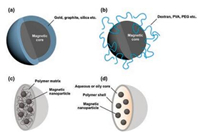
|
Download:
|
| Fig. 3. Schematic representation of the stabilization of MNPs by surface coating with (a) inorganic, (b) organic materials, (c) encapsulation into nanospheres or (d) nanocapsules. Reproduced with permission [120]. Copyright 2012, American Chemical Society. | |
5.1. Organic coating
The surfaces of the nanoparticles are oftenpassivated byadding surfactants or polymers during or after the synthesis to avoid aggregation. Papell dispersed nanoparticles and keep ferrofluids in a steady colloidal condition via electrostatic repulsion or steric repulsion which were invented in 1965 [121]. Controlling surface charge [122] and using specific surfactants [123] are the major measures to stabilize ferrofluids. Thus, a single layer or multilayer can be formed through the physical adsorption or chemical anchorage of the surfactants or polymers on magnetic nanoparticles [124, 125]. Therefore, the magnetic particles achieve a balance in suspension by steric repulsion. Polymers can anchor to the surface of magnetite [126]. Use of suitable polymer coating including poly(aniline), poly(alkylcyanoacrylates), poly(pyrrole), poly(methylidene malonate), and polyesters, such as poly(lactic acid), poly(glycolic acid), poly(e-caprolactone), and with certain biocompatible polymers, their copolymers [127, 128]. Surfacemodified magnetic nanoparticles concentratively investigated for magnetic-field-directed drug targeting, and are used as contrast agents [129]. Reactive metal magnetic nanoparticles are easily corroded by acidic and alkal solution. Thus, reactive magnetic nanoparticles are not very suitable to be protected by polymer coating. The temperature is higher and the relatively intrinsic stability of the coating is lower. Therefore, it is of great importance for developing other methods to protect magnetic nanoparticles against deterioration.
5.2. Inorganic coatingCarbon coating are used to protected magnetic nanoparticles which has received increasing attention recently carbon-based materials have much thermal stability, higher chemical, biocompatibility than polymer or silica. After the discovery of fullerenes, It was found that the KrStschmer arc-discharge process can generate carbon-encapsulated metal or carbide-nanocrystallites metal [130]. Since then, graphitized carbon structures such as carbon onions and carbon nanotubes are formed under laser ablation, arcdischarge and electron irradiation in the presence of metal nanoparticles (Au, Ni, Cr, etc.) [131, 132]. It shows that welldeveloped carbon-coated magnetic nanoparticles is crucial for some applications, which provide an perfect barrier against acid erosion and oxidation [133]. And also, graphitic carbon coating has high stability against oxidation and acid leaching in the metallic state. Gedanken and co-workers claimed that a carbon shell which is formed on the nanoparticle surface results in high stability. They reported that the sonochemical procedure results in air-stable cobalt nanoparticles [134]. However, the obtained particles are polydispersity. Johnson et al. pyrolysis iron stearate at 900℃ directly and prepared carbon-coated magnetic Fe3C and Fe nanoparticles under argon atmosphere [135]. Obtained magnetic nanoparticles are stable and it can be up to 400℃ under air with carbon coating. It is a perfect single-step process to scale up saltconversionprocess directly. However, a broad size distribution that the nanoparticles' diameter ranges from 20nm to 200nm, while produced by this method and 20 to 80 graphene layers covered the cores. Carbon-coated magnetic nanoparticles are thermally stabile and they have high stability against acid leaching, oxidation. However, particles are often difficult to keep isolated form, owing to the lack of mechanism. At present, it is one of the challenges in this field to find effective synthetic methods for dispersibility of the carbon-coated nanoparticles.
6. Nanomedical applications 6.1. BioimagingWith the help of molecular imaging, after transplantation, researchers can know some important performances of the therapeutic cells, such as the principle, stability and function [136, 137]. Imaging techniques consists of three parts, optical imaging, MRI, and radionuclide imaging respectively. In order to label cells and make a signal to separate it from the host tissue, we usually use contrast agents to achieve these targets. Due to the wavelength of the applied light, the penetration depth (< 5 μm) in human being's body restricts the styles of optical imaging. In medical imaging, the best sensitivity belongs to Radionuclide imaging styles. But, there still has some imperfections, for example, it cannot track the cell in lengthways, anatomical information is very less and endangers human health. In a clinical research, De Vries et al. reported that through MRI without invasive, we can track therapeutic cells in patients effectively [138]. However, there still has limitation, through MRI to track cell only can help understand the distribution of organisms of transplanted therapeutic cells. The most important point is that we cannot get the message of its function and destiny after engraftment of stem cells [139]. In order to solve this problem, researchers wants to develop a multifunctional platform [140] by put MRI with sensors, so that they can get the information of location and function.
6.2. In vitro bioseparationFor MNPs, to separate the cell or protein extracorporeal is another meaning application. Magnetic separation techniques several advantages compared with traditional separation methods. This process is not complex and very cheap. Fan [141] and Xu [142] have developed functionalized MNPs used for the separation of protein antibiotics. MNPs coated with Dopamine have high capacity and could test vancomycin-resistant enterococci or Gram-positive bacteria at light density [143]. Functional groups can be synthesized by surface exchange reactions [144, 145] and organic overlay precipitation [146]. Due to the doughty interaction between metal oxides, organosilanes can be applied to attach functional groups to MNPs. synthesizing the silica coating is divided into two steps: (ⅰ) preparation of silica by sol-gel hydrolysis and condensation [147] and (ⅱ) the control of the silica coating [148]. Magnetic capsule, MNPs coated with phospholipids, have been applied to classification of proteins [149].
6.3. Drug deliveryCharacteristics of MNPs is largely determined by the dimension. After administration, large particles greater than 200 nm are facility removed by the splenic macrophage system cells, thereby reducing the times of blood circulation. Small particle diameter less than 10 nm are quickly removed by extravasation and renal clearance. Particles with diameters of 10–100 nm are optimal for intravenous injection and have the longest cycle time. Targeting of MNPs as a carrier for magnetic drug therapy is an effective method to avoid the side effects of traditional chemotherapy, starch phosphate groups covered iron oxide nanoparticle [150]. Alexiou et al. have shown that the accumulation of nanoparticles induced by high magnetic field gradient at the tumor site [151].
6.4. HyperthermiaFerrofluids are effective materials and can be used in the treatment of diagnosis. Their application to hyperthermia therapy was first conceived in the pioneering research of Jordan et al. [152]. The research has proved that the super paramagnetic crystals can be efficiently absorbed and transformed the magnetic energy into heat. Cancer cells are more sensitive to a temperature raise than normal cells. The more useful methods involve the submission of a patient's electromagnetic wave to a few 100 MHz frequencies. The less side-effect means involve irradiating the lesion area with an external resonant microwave dipole array [153]. Clinical and clinical data indicate that hyperthermia combined with radiotherapy is viable. Selective deactivation of tumor cells in vitro has been verified. Theoretically, the superparamagnetic crystals should be enclosed with a drug in a liposome. The drug should be released in large quantities and selectively in the magnetic field. All in all, superparamagnetic colloids are extremely hopeful materials for hyperthermia treat. However, the application of this new field needs to be improved in the reproducibility and particle size control.
6.5. Disease therapyTraditional drug delivery is always restricted to bad functionalities to target sites and blocked drug dispersion through biobarrier, resulting in reduced drug activity and large probability of side effects [154-157]. These problems can be solved by using nano-device transport and drug packaging. Vector-mediated drug delivery was under great concern in recent years for reason that this methods leads to improved pharmacokinetics and pharmacodynamics of the drug to improved function effectiveness and stability [158-161]. Drug packaging into a nanocarrier has the capacity to extend the systemic circulation of the drug, and increase the probability that the drug reach unhealthy cells [162-166]. Therefore, in order to perfect the activity of therapeutic, increase the half-life and the exposure of the drug with MNPsencapsulated will be necessarily. As a result, being a fraction of the active drug-targeting strategies, drugs can deliver to human body selectively with the help of stimuli-sensitive carriers. We can change the physical properties when they suffered an external stimulus exposured by using colloidal systems, so that the drug accumulation can be reinforced at the expected site of delivery.
Under this circumstance, due to MNPs' specific magnetic responsiveness, MNPs can be the hopeful stimuli-sensitive drug carriers. In other words, those nano-devices can be focused at the intended site, so that before releasing the drugs, they can be kept at a proper place for some time researchers set up before, and as a result significantly reduce the drug-associated side effects caused by nonspecific distribution [167, 168]. Ordinarily, the MNPs with different size have different effect, particles smaller than 100 nm possess excellent tissue permeability properties, so that those MNPs usually be used to give out heat, and the lager particles have better sensitivity to magnetic guidance, therefore, those MNPs usually be used as magnetic targets [169].
Magnetic nanoparticles-mediated targeted magnetic hyperthermia (TMH) is a rapid-developing tumor therapy in vivo. Based on its curative effect, high safety and less side effects, the therapy may widely receive enormous attention in further clinical applications. However, it is rather challenging in the MNPs-based passive or active targeted hyperthermia therapy by intravenously administration in vivo and their further enhanced therapy efficacy.
7. ConclusionIn this paper, we have presented the special features of MNPs and the common methods applied for the synthesis of MNPs with precisely control of shape and size. We have discussed ways in which MNPs are stabilized and functionalized for nanomedical utilization. Lastly, we have outlined application of MNPs for biomedicine, such as drug delivery, hyperthermia, disease therapy and MRI. These illustrated examples further demonstrate that MNPs are indeed promising for nanomedical utilization.
AcknowledgmentsThe authors would like to thank the financial support from the National Natural Science Foundation of China (No. 81773642), Guangdong-Hong Kong Technology Cooperation Fund (No. 2017A050506016), the Science and Technology Planning Program of Guangzhou City, China (No. 2017A020214012), Natural Science Foundation of the Jiangsu Higher Education Institutions (No. 17KJB430019), Natural Science Foundation of the Jiangsu Province (No. SBK2018041659), Jiangsu Key Laboratory of Green Process Equipment (No. GPE201702), and GF Scientific Research Project of Nanjing Tech University.
| [1] |
Q. Mou, Y. Ma, X. Zhu, D. Yan, J. Control. Release 230 (2016) 34-44. DOI:10.1016/j.jconrel.2016.03.037 |
| [2] |
J. Tian, C. Yao, W. Yang, et al., Chin. Chem. Lett. 28 (2017) 798-806. DOI:10.1016/j.cclet.2017.01.010 |
| [3] |
C. Yao, J. Tian, H. Wang, et al., Chin. Chem. Lett. 28 (2017) 893-899. DOI:10.1016/j.cclet.2017.01.005 |
| [4] |
N. Chen, Y. Huang, Y. Wang, Biomaterials 35 (2014) 9709-9718. DOI:10.1016/j.biomaterials.2014.08.017 |
| [5] |
Z. Chen, Z. Chen, A. Zhang, et al., Biomater. Sci.-UK 4 (2016) 922-932. DOI:10.1039/C6BM00070C |
| [6] |
Z. Chen, A. Zhang, X. Wang, et al., J. Nanomater. 8 (2017) 1-13. DOI:10.3390/nano8010001 |
| [7] |
Y. Xiao, F.F. An, J. Chen, S. Xiong, X.H. Zhang, J. Mater. Chem. B 22 (2018) 1-17. |
| [8] |
J. Panyam, V. Labhasetwar, Adv. Drug Deliv. Rev. 55 (2003) 329. DOI:10.1016/S0169-409X(02)00228-4 |
| [9] |
S.M. Moghimi, A.C. Hunter, J.C. Murray, Pharmacol. Rev. 53 (2001) 283-318. |
| [10] |
L. Hao, X.L. Liu, J.T. Wang, et al., Chin. Chem. Lett. 27 (2016) 783-788. DOI:10.1016/j.cclet.2016.01.021 |
| [11] |
Q.L. Chen, Y.Y. Yang, X. Lin, W. Ma, G. Chen, W.L. Li, et al., Chem. Commun. 54 (2018) 8713-8716. DOI:10.1039/C8CC04783A |
| [12] |
X. Wang, Z. Guo, Chem. Soc. Rev. 42 (2013) 202-224. DOI:10.1039/C2CS35259A |
| [13] |
J. Chen, C. Gao, Y. Zhang, et al., J. Nanosci. Nanotechnol. 17 (2017) 1-17. DOI:10.1166/jnn.2017.12932 |
| [14] |
X. Mou, Z. Ali, S. Li, N. He, Nanosci J., Nanotechnology 15 (2015) 54. DOI:10.1166/jnn.2015.9585 |
| [15] |
H.S. Johal, T. Garg, G. Rath, A.K. Goyal, Drug Deliv. 23 (2014) 1. |
| [16] |
S.W. Tan, H.J. Wang, K.H. Tu, H.L. Jiang, L.Q. Wang, Chin. Chem. Lett. 22 (2011) 1123-1126. DOI:10.1016/j.cclet.2011.04.005 |
| [17] |
T. Kuang, L. Chang, F. Chen, et al., Carbon 105 (2016) 305-313. DOI:10.1016/j.carbon.2016.04.052 |
| [18] |
C. Yu, L. Qian, J. Ge, et al., Angew. Chem. 55 (2016) 9272-9276. DOI:10.1002/anie.201602188 |
| [19] |
C. Yu, L. Qian, M. Uttamchandani, L. Li, S.Q. Yao, Angew. Chem. 54 (2015) 10574-10578. DOI:10.1002/anie.201504913 |
| [20] |
M. Yu, F. Guo, F. Tan, N. Li, J. Control. Release 215 (2015) 91-100. DOI:10.1016/j.jconrel.2015.08.003 |
| [21] |
Y. Liu, J.K. Yu, N. Siriwon, et al., Biotechnol. Bioeng. 115 (2018) 1403-1415. DOI:10.1002/bit.v115.6 |
| [22] |
H. Gao, Q. Zhang, Z. Yu, Q. He, Curr. Pharm. Biotechno. 15 (2014) 210-219. DOI:10.2174/1389201015666140617092552 |
| [23] |
Z.Q. Yu, C. Zheng, Q.L. Chen, et al., Biomater. Sci.-UK 4 (2015) 365-374. |
| [24] |
W. Liao, Z. Rong, C. Dong, Z. Yu, J. Ren, Int. J. Nanomed. Nanosurg. 11 (2016) 1305-1321. |
| [25] |
Z.Q. Yu, Q. Xu, C.B. Dong, et al., Curr. Pharm. Design 21 (2015) 4342-4354. DOI:10.2174/1381612821666150901104821 |
| [26] |
M. Yu, F. Guo, J. Wang, F. Tan, N. Li, Biomaterials 79 (2016) 25-35. DOI:10.1016/j.biomaterials.2015.11.049 |
| [27] |
B.R. Schroeder, M.I. Ghare, C. Bhattacharya, et al., J. Am. Chem. Soc. 136 (2015) 13641-13656. |
| [28] |
M. Madathil, C. Bhattacharya, Z. Yu, et al., Biochemistry 53 (2014) 6800. DOI:10.1021/bi501102z |
| [29] |
C. Bhattacharya, Z. Yu, M.J. Rishel, S.M. Hecht, Biochemistry 53 (2014) 3264-3266. DOI:10.1021/bi500482q |
| [30] |
C.F. He, S.H. Wang, Y.J. Yu, et al., Cancer Biol. Med. 13 (2016) 299-312. DOI:10.20892/j.issn.2095-3941.2016.0052 |
| [31] |
Z. Chen, A. Zhang, Z. Yang, et al., Curr. Org. Chem. 20 (2016) 1813-1819. DOI:10.2174/1385272820666160202004348 |
| [32] |
M. Xue, Y.W. Yang, Chin. Chem. Lett. 28 (2017) 1135-1143. DOI:10.1016/j.cclet.2017.03.026 |
| [33] |
C. Hu, Z. Chen, S. Wu, et al., Chin. Chem. Lett. 28 (2017) 1905-1909. DOI:10.1016/j.cclet.2017.07.020 |
| [34] |
A. Curtis, C. Wilkinson, Mater. Today 4 (2001) 22-28. |
| [35] |
F.F. An, W. Cao, X.J. Liang, Adv. Healthc. Mater. 3 (2015) 1162-1181. |
| [36] |
F.F. An, X.H. Zhang, Theranostics 7 (2017) 3667-3689. DOI:10.7150/thno.19365 |
| [37] |
N. Lewinski, V. Colvin, R. Drezek, Small 4 (2008) 26-49. |
| [38] |
J. Chatterjee, Y. Haik, C.J. Chen, J. Magn. Magn. Mater. 257 (2003) 113-118. DOI:10.1016/S0304-8853(02)01066-1 |
| [39] |
P. Tartaj, S. Veintemillas-Verdaguer, C.J. Serna, J. Phys. D:Appl. Phys. 36 (2003) R182. DOI:10.1088/0022-3727/36/13/202 |
| [40] |
Z. Chen, M. Cong, J. Hu, Z. Yang, Z. Chen, Sci. Adv. Mater. 8 (2016) 1231-1241. DOI:10.1166/sam.2016.2719 |
| [41] |
S. Laurent, D. Forge, M. Port, et al., Chem. Rev. 108 (2008) 2064-2110. DOI:10.1021/cr068445e |
| [42] |
J.R. McCarthy, K.A. Kelly, E.Y. Sun, R. Weissleder, Nanomedicine 2 (2007) 153-167. DOI:10.2217/17435889.2.2.153 |
| [43] |
O. Veiseh, J.W. Gunn, M. Zhang, Adv. Drug Deliver. Rev. 62 (2010) 284-304. DOI:10.1016/j.addr.2009.11.002 |
| [44] |
N. Sanvicens, M.P. Marco, Trends Biotechnol. 26 (2008) 425-433. DOI:10.1016/j.tibtech.2008.04.005 |
| [45] |
M. Ferrari, Nat. Rev. Cancer 5 (2005) 161-171. DOI:10.1038/nrc1566 |
| [46] |
D. Li, C.N. Lin, S.Z. Zhan, C.L. Ni, Chin. Chem. Lett. 28 (2017) 1424-1428. DOI:10.1016/j.cclet.2017.03.027 |
| [47] |
T. Kuang, F. Chen, L. Chang, et al., Chem. Eng. J. 307 (2016) 1017-1025. |
| [48] |
J.M. Perez, L. Josephson, T. O'Loughlin, D. Högemann, R. Weissleder, Nat. Biotechnol. 20 (2002) 816-820. DOI:10.1038/nbt720 |
| [49] |
M.M. Miller, G.A. Prinz, S.F. Cheng, S. Bounnak, Appl. Phys. Lett. 81 (2002) 2211-2213. DOI:10.1063/1.1507832 |
| [50] |
S. Sun, C.B. Murray, D. Weller, L. Folks, A. Moser, Science 31 (2000) 1989-1992. |
| [51] |
D. Weller, A. Moser, Ieee Transl. J. Magn. Jpn. 35 (1999) 4423-4439. DOI:10.1109/20.809134 |
| [52] |
R.H. Dee, P IEEE 96 (2008) 1775-1785. DOI:10.1109/JPROC.2008.2004311 |
| [53] |
D.W. Elliott, W.X. Zhang, Environ. Sci. Technol. 35 (2001) 4922. DOI:10.1021/es0108584 |
| [54] |
B. Thiesen, A. Jordan, Int. J. Hyperthermia 24 (2008) 467-474. DOI:10.1080/02656730802104757 |
| [55] |
Q.A. Pankhurst, J. Connolly, S.K. Jones, J. Dobson, J. Phys. D:Appl. Phys. 36 (2003) R167. DOI:10.1088/0022-3727/36/13/201 |
| [56] |
C. Sun, J.S. Lee, M. Zhang, Adv. Drug Deliver. Rev. 60 (2008) 1252-1265. DOI:10.1016/j.addr.2008.03.018 |
| [57] |
L. Frullano, T.J. Meade, J. Biol. Inorg. Chem. 12 (2007) 939-949. DOI:10.1007/s00775-007-0265-3 |
| [58] |
Z. Li, L. Wei, M. Gao, H. Lei, Adv. Mater. 17 (2005) 1001-1005. |
| [59] |
R. Weissleder, A. Moore, U. Mahmood, et al., Nat. Med. 6 (2000) 351-354. DOI:10.1038/73219 |
| [60] |
V.P. Torchilin, Adv. Drug Deliver. Rev. 64 (2012) 302-315. DOI:10.1016/j.addr.2012.09.031 |
| [61] |
B. Bonnemain, J. Drug Target. 6 (1998) 167-174. DOI:10.3109/10611869808997890 |
| [62] |
D. Jiles, Acta Mater. 51 (2003) 5907-5939. DOI:10.1016/j.actamat.2003.08.011 |
| [63] |
D.L. Huber, Small 1 (2005) 482-501. |
| [64] |
S. Guo, S. Zhang, S. Sun, Cheminform 52 (2013) 8526-8544. |
| [65] |
A.S. Arbab, L.A. Bashaw, B.R. Miller, et al., Radiology 229 (2003) 838-846. DOI:10.1148/radiol.2293021215 |
| [66] |
C. Kittel, Phys. Rev. 70 (1946) 965-971. DOI:10.1103/PhysRev.70.965 |
| [67] |
G. Herzer, Ieee Transl. J. Magn. Jpn. 26 (1990) 1397-1402. DOI:10.1109/20.104389 |
| [68] |
C.B.M. And, C.R. Kagan, M.G. Bawendi, Annu. Rev. Mater. Res. 30 (2000) 545-610. |
| [69] |
Y. Yin, A.P. Alivisatos, Nature 437 (2005) 664-670. DOI:10.1038/nature04165 |
| [70] |
D.V. Talapin, J.S. Lee, M.V. Kovalenko, E.V. Shevchenko, Chem. Rev. 110 (2010) 389-458. DOI:10.1021/cr900137k |
| [71] |
M. Cargnello, T.R. Gordon, C.B. Murray, Chem. Rev. 114 (2014) 9319-9345. DOI:10.1021/cr500170p |
| [72] |
A.K. Gupta, R.R. Naregalkar, V.D. Vaidya, M. Gupta, NanomedicineUK 2 (2007) 23-39. DOI:10.2217/17435889.2.1.23 |
| [73] |
A.G. Kolhatkar, A.C. Jamison, D. Litvinov, R.C. Willson, T.R. Lee, Int. J. Mol. Sci. 14 (2013) 15977-16009. DOI:10.3390/ijms140815977 |
| [74] |
R. Yu, C.F. Jiang, W. Chu, M.F. Ran, W.J. Sun, Chin. Chem. Lett. (2017) 302-306. |
| [75] |
J. Lim, C. Lanni, E.R. Evarts, et al., ACS Nano 5 (2011) 217-226. DOI:10.1021/nn102383s |
| [76] |
J. Faraudo, J. Camacho, Colloid Polym. Sci. 288 (2010) 207-215. DOI:10.1007/s00396-009-2107-z |
| [77] |
E.P. Furlani, Materials 3 (2010) 2412-2446. DOI:10.3390/ma3042412 |
| [78] |
Y. Sahoo, A. Goodarzi, M.T. Swihart, et al., J. Phys. Chem. B 109 (2014) 3879-3885. |
| [79] |
X. Batlle, A. Labarta, J. Phys. D:Appl. Phys. 35 (2002) 15-42. |
| [80] |
S. Peng, C. Wang, J. Xie, S.H. Sun, J. Am. Chem. Soc. 128 (2006) 10676-10677. DOI:10.1021/ja063969h |
| [81] |
J.L. Viota, J.D.G. Durán, F. González-Caballero, A.V. Delgado, J. Magn. Magn. Mater. 314 (2007) 80-86. DOI:10.1016/j.jmmm.2007.02.002 |
| [82] |
J.P. Jolivet, C. Chanéac, E. Tronc, Cheminform 35 (2004) 477-483. |
| [83] |
J. Park, K. An, Y. Hwang, et al., Nat. Mater. 3 (2004) 891-895. DOI:10.1038/nmat1251 |
| [84] |
F.X. Redl, C.T. Black, G.C. Papaefthymiou, et al., Chem. Soc. 126 (2004) 14583-14599. DOI:10.1021/ja046808r |
| [85] |
J. Park, E. Lee, N.M. Hwang, et al., Angew. Chem. 44 (2005) 2873-2877. |
| [86] |
D. Farrell, S.A. Majetich, J.P. Wilcoxon, J. Phys. Chem. B 107 (2003) 11022-11030. DOI:10.1021/jp0351831 |
| [87] |
Y. Li, M. Afzaal, P. O'Brien, J. Mater. Chem. 16 (2006) 2175-2180. DOI:10.1039/b517351e |
| [88] |
F. Chen, Q. Gao, G. Hong, J. Ni, J. Magn. Magn. Mater. 320 (2008) 1775-1780. DOI:10.1016/j.jmmm.2008.02.117 |
| [89] |
H. Itoh, T. Sugimoto, J. Colloid Interf. Sci. 265 (2003) 283-295. DOI:10.1016/S0021-9797(03)00511-3 |
| [90] |
C. Cannas, D. Gatteschi, A. Musinu, G. Piccaluga, C. Sangregorio, J. Phys. Chem. B 102 (1998) 7721-7726. DOI:10.1021/jp981355w |
| [91] |
D. Langevin, Annu. Rev. Phys. Chem. 43 (1992) 341-369. DOI:10.1146/annurev.pc.43.100192.002013 |
| [92] |
S.P. Moulik, B.K. Paul, Curr. Sci. India 80 (2001) 990-1001. |
| [93] |
A.K. Gupta, M. Gupta, Biomaterials 26 (2005) 3995-4021. DOI:10.1016/j.biomaterials.2004.10.012 |
| [94] |
K.S. Suslick, Science 247 (1990) 1439. DOI:10.1126/science.247.4949.1439 |
| [95] |
J.H. Bang, K.S. Suslick, Adv. Mater. 22 (2010) 1039. DOI:10.1002/adma.200904093 |
| [96] |
B.W. Hojatollah Vali, Y.L. Li, S. Kelly Sears, et al., Natl. Acad. Sci. 101 (2004) 16121-16126. DOI:10.1073/pnas.0404040101 |
| [97] |
A. Scheffel, M. Gruska, D. Faivre, et al., Nature 440 (2006) 110-114. DOI:10.1038/nature04382 |
| [98] |
K. Niemirowicz, H. Car, A. Sadowska, et al., J. Biomed. Nanotechnol. 13 (2017) 665-677. DOI:10.1166/jbn.2017.2363 |
| [99] |
I.L. Medintz, H.T. Uyeda, E.R. Goldman, H. Mattoussi, Nat. Mater. 4 (2005) 435. DOI:10.1038/nmat1390 |
| [100] |
X. Michalet, F.F. Pinaud, L.A. Bentolila, et al., Science 307 (2005) 538. DOI:10.1126/science.1104274 |
| [101] |
W.S. Seo, J.H. Lee, X. Sun, et al., Nat. Mater. 5 (2006) 971-976. DOI:10.1038/nmat1775 |
| [102] |
J. Shin, R.M. Anisur, M.K. Ko, et al., Angew. Chem. 121 (2009) 327-330. DOI:10.1002/ange.v121:2 |
| [103] |
B.L. Frankamp, N.O. Fischer, R. Hong, S. Srivastava, V.M. Rotello, Chem. Mater. 18 (2006) 956-959. DOI:10.1021/cm052205i |
| [104] |
B. Dubertret, P. Skourides, D.J. Norris, et al., Science 298 (2002) 1759-1762. DOI:10.1126/science.1077194 |
| [105] |
X. Michalet, F. Pinaud, L. Bentolila, et al., Science 307 (2005) 538-544. DOI:10.1126/science.1104274 |
| [106] |
A.G. Martínez, J.P. Juste, L.M. Marzán, Adv. Mater. 22 (2010) 1182-1195. DOI:10.1002/adma.v22:11 |
| [107] |
Y. Lu, Y. Yin, B.T. Mayers, Y. Xia, Nano Lett. 2 (2002) 183-186. DOI:10.1021/nl015681q |
| [108] |
Y. Piao, J. Kim, H.B. Na, et al., Nat. Mater. 7 (2008) 242-247. DOI:10.1038/nmat2118 |
| [109] |
T.J. Yoon, H. Lee, H. Shao, S.A. Hilderbrand, R. Weissleder, Adv. Mater. 23 (2011) 4793-4797. DOI:10.1002/adma.201102948 |
| [110] |
Y. Kobayashi, M. Horie, M. Konno, et al., J. Phys. Chem. B 107 (2003) 7420-7425. DOI:10.1021/jp027759c |
| [111] |
W. Zhao, J. Gu, L. Zhang, H. Chen, J. Shi, J. Am. Chem. Soc. 127 (2005) 8916-8917. DOI:10.1021/ja051113r |
| [112] |
U. Schwertmann, R.M. Cornell, Clay Miner. 27 (2000) 393. |
| [113] |
B. Vincent, J. Edwards, S. Emmett, A. Jones, Colloids Surf. 18 (1986) 261-281. DOI:10.1016/0166-6622(86)80317-1 |
| [114] |
B. Derjaguin, Physicochim Acta, USSR 14 (1941) 633-662. |
| [115] |
L.E. Euliss, S.G. Grancharov, S. O'Brien, et al., Nano Lett. 3 (2003) 1489-1493. DOI:10.1021/nl034472y |
| [116] |
M. Kim, Y. Chen, Y. Liu, X. Peng, Adv. Mater. 17 (2005) 1429-1432. |
| [117] |
L.H. Geng, L. Li, H.Y. Mi, et al., ACS Appl. Mater. Inter. 9 (2017) 21071-21076. DOI:10.1021/acsami.7b05127 |
| [118] |
N.S. Sobal, M. Hilgendorff, H. Möhwald, et al., Nano Lett. 2 (2002) 621-624. DOI:10.1021/nl025533f |
| [119] |
D.K. Kim, M. Mikhaylova, Y. Zhang, M. Muhammed, Chem. Mater. 15 (2003) 1617-1627. DOI:10.1021/cm021349j |
| [120] |
L.H. Reddy, J.L. Arias, J. Nicolas, P. Couvreur, Chem. Rev. 112 (2012) 5818-5878. DOI:10.1021/cr300068p |
| [121] |
P.S. Stephen, Google Patents (1965). |
| [122] |
R. Massart, IEEE T M 17 (1981) 1247-1248. DOI:10.1109/TMAG.1981.1061188 |
| [123] |
M. De Cuyper, M. Joniau, Langmuir 7 (1991) 647-652. DOI:10.1021/la00052a010 |
| [124] |
L. Shen, P.E. Laibinis, T.A. Hatton, Langmuir 15 (1999) 447-453. DOI:10.1021/la9807661 |
| [125] |
M.H. Sousa, F.A. Tourinho, J. Depeyrot, G.J. da Silva, M.C.F. Lara, J. Phys. Chem. B 105 (2001) 1168-1175. DOI:10.1021/jp0039161 |
| [126] |
R.M. Cornell, U. Schwertmann, Mineral. Mag. 61 (1997) 740-741. DOI:10.1180/minmag.1997.061.408.20 |
| [127] |
M. Wan, J. Li, J. Polym. Sci. Polym. Chem. Ed. 36 (1998) 2799-2805. |
| [128] |
G. Barratt, Cell. Mol. Life Sci. 60 (2003) 21-37. DOI:10.1007/s000180300002 |
| [129] |
A.F. Thünemann, D. Schütt, L. Kaufner, U. Pison, H. Möhwald, Langmuir 22 (2006) 2351-2357. DOI:10.1021/la052990d |
| [130] |
J.H.J. Scott, S.A. Majetich, Phys. Rev. B 52 (1995) 12564. DOI:10.1103/PhysRevB.52.12564 |
| [131] |
T. Hayashi, S. Hirono, M. Tomita, S. Umemura, J.J. Delaunay, Magnetic thin films of cobalt nanocrystals encapsulated in graphite-like carbon, MRS Proceedings, Cambridge Univ. Press, 1997, p. 33.
|
| [132] |
R. Nesper, A. Ivantchenko, F. Krumeich, Adv. Funct. Mater. 16 (2006) 296-305. |
| [133] |
H.B. Chan, B.L. Ellis, H.L. Sharma, et al., Adv. Mater. 16 (2004) 144-149. |
| [134] |
S.I. Nikitenko, Y. Koltypin, O. Palchik, et al., Angew. Chem. Int. Ed. 40 (2001) 4447-4449. DOI:10.1002/1521-3773(20011203)40:23<4447::AID-ANIE4447>3.0.CO;2-J |
| [135] |
J. Geng, D. Jefferson, B. Johnson, Chem. Commun. 23 (2004) 2442-2443. |
| [136] |
L. Chang, J. Hu, F. Chen, et al., Nanoscale 8 (2016) 3181-3206. DOI:10.1039/C5NR06694H |
| [137] |
Y. Ye, J. Xing, L. Zeng, et al., J. Biomed. Nanotechnol. 13 (2017) 980-988. DOI:10.1166/jbn.2017.2414 |
| [138] |
I.J.M. de Vries, W.J. Lesterhuis, J.O. Barentsz, et al., Nat. Biotechnol. 23 (2005) 1407-1413. DOI:10.1038/nbt1154 |
| [139] |
Y. Gao, Y. Cui, J. Chan, C. Xu, Am. J. Nucl. Med. Mol. Imaging 3 (2013) 232. |
| [140] |
C. Xu, J. Xie, D. Ho, et al., Angew. Chem. 120 (2008) 179-182. |
| [141] |
J. Fan, J. Lu, R. Xu, R. Jiang, Y. Gao, J. Colloid Interf. Sci. 266 (2003) 215-218. DOI:10.1016/S0021-9797(03)00570-8 |
| [142] |
C. Xu, K. Xu, H. Gu, et al., J. Am. Chem. Soc. 126 (2004) 9938-9939. DOI:10.1021/ja0464802 |
| [143] |
H. Gu, P.L. Ho, K.W. Tsang, L. Wang, B. Xu, J. Am. Chem. Soc. 125 (2003) 15702-15703. DOI:10.1021/ja0359310 |
| [144] |
K.C. Ho, P.J. Tsai, Y.S. Lin, Y.C. Chen, Anal. Chem. 76 (2004) 7162-7168. DOI:10.1021/ac048688b |
| [145] |
J. Lu, J. Fan, R. Xu, et al., J. Colloid Interf. Sci. 258 (2003) 427-431. DOI:10.1016/S0021-9797(02)00152-2 |
| [146] |
Y. Wang, J.F. Wong, X. Teng, X.Z. Lin, H. Yang, Nano Lett. 3 (2003) 1555-1559. DOI:10.1021/nl034731j |
| [147] |
W. Stöber, A. Fink, E. Bohn, J. Colloid Interf. Sci. 26 (1968) 62-69. DOI:10.1016/0021-9797(68)90272-5 |
| [148] |
X. Gao, K.K. Yu, K.Y. Tam, S.C. Tsang, Chem. Commun. (Camb.) (2003) 2998-2999. |
| [149] |
S. Bucak, D.A. Jones, P.E. Laibinis, T.A. Hatton, Biotechnol. Progr. 19 (2003) 477-484. DOI:10.1021/bp0200853 |
| [150] |
Y. Wang, X. Zhao, W. Du, et al., J. Biomed. Nanotechnol. (2017) 1673-1681. |
| [151] |
C. Alexiou, R.J. Schmid, R. Jurgons, et al., Eur. Biophys. J. Biophy. 35 (2006) 446-450. DOI:10.1007/s00249-006-0042-1 |
| [152] |
A. Jordan, P. Wust, H. Fähling, et al., Int. J. Hyperthermia 25 (2009) 499-511. DOI:10.3109/02656730903287790 |
| [153] |
M.M. Paulides, J.F. Bakker, A.P. Zwamborn, G.C. Van Rhoon, Int. J. Hyperthermia 23 (2007) 59-67. DOI:10.1080/02656730601150522 |
| [154] |
C. Kang, Y. Sun, J. Zhu, et al., Curr. Drug Metab. 17 (2016) 745-754. DOI:10.2174/1389200217666160728152939 |
| [155] |
J. Xie, Z. Yang, C. Zhou, et al., Biotechnol. Adv. 34 (2016) 343-353. DOI:10.1016/j.biotechadv.2016.04.002 |
| [156] |
Z. Chen, Z. Chen, A. Zhang, et al., Biomater. Sci. 4 (2016) 922-932. DOI:10.1039/C6BM00070C |
| [157] |
C. Zhou, Z. Yang, L. Teng, Curr. l 15 (2014) 829-838. |
| [158] |
Z. Yang, J. Xie, J. Zhu, et al., J. Control. Release 243 (2016) 160-171. DOI:10.1016/j.jconrel.2016.10.008 |
| [159] |
X. Yang, S. Yang, H. Chai, et al., PLoS One 10 (2015) e0136649. DOI:10.1371/journal.pone.0136649 |
| [160] |
Z. Yang, B. Yu, J. Zhu, et al., Nanoscale 6 (2014) 9742-9751. DOI:10.1039/C4NR01510J |
| [161] |
J. Xie, L. Teng, Z. Yang, et al., Biomed Res. Int. 2013 (2013) 710502. |
| [162] |
B. Yu, X. Wang, C. Zhou, et al., Pharm. Res. 31 (2014) 2685-2695. DOI:10.1007/s11095-014-1366-7 |
| [163] |
X. Wang, X. Huang, Z. Yang, et al., Curr. Pharm. Biotechnol. 15 (2014) 839-846. DOI:10.2174/1389201015666141031105234 |
| [164] |
L.L. Sha, Z.F. Chen, Z. Chen, A.L. Zhang, Z.G. Yang, Int.J.Polym.Sci. (2016) 6869154. |
| [165] |
Z. Chen, A.L. Zhang, Z.G. Yang, et al., Curr. Org. Chem. 20 (2016) 1813-1819. DOI:10.2174/1385272820666160202004348 |
| [166] |
J. Song, J. Xie, C. Li, et al., Inter. J. Pharmaceut. 472 (2014) 296-303. DOI:10.1016/j.ijpharm.2014.06.033 |
| [167] |
A. Ibrahim, P. Couvreur, M. Roland, P. Speiser, J. Pharm. Pharmacol. 35 (1983) 59-61. DOI:10.1111/jphp.1983.35.issue-1 |
| [168] |
N. Kohler, C. Sun, J. Wang, M. Zhang, Langmuir 21 (2005) 8858-8864. DOI:10.1021/la0503451 |
| [169] |
J.L. Arias, Molecules 13 (2008) 2340-2369. DOI:10.3390/molecules13102340 |
 2018, Vol. 29
2018, Vol. 29 



