b Department of Chemistry, Fudan University, Shanghai 200433, China
π-Conjugated polymers normally possess distinct electrochemical properties, conductivity, and photocatalytic activity. With the specific stimuli-response to light and electrical stimuli, it can be applied into various optoelectrical devices [1-3]. It has always been a heated research focus as reported in some recent progresses concerning self-assembly and functional conjugated polymers [4-8]. Polydiacetylenes (PDAs) are among one of the most interested π-conjugated materials, since many relevant works have been focused on the polymerization of alkyl diacetylene under light irradiation [9, 10].
The organized alignment and compact adjacent distance of the monomers or a certain template for synthesis can result in an anisotropic polymer with better structural specificity and optoelectronic properties. It is obvious that the molecular preorganization through controlled aggregation or template-grafting can promote the topochemical polymerization [11, 12]. Particularly, the distance between two diacetylene units (usually less than 5 Å) is concerned to be equally important for topochemical polymerization in crystals or other self-assembled structures [13]. The first synthesis with precise preorganization of PDA within crystals was conducted by Wegner and coworkers [14]. Later, various research groups have been devoted to the design of the fine structures of molecular assemblies like crystals, micelles, Langmuir-Blodgett films, etc. to prepare the parallel packing mode for topochemical polymerization [15-17]. Since the morphology of monomers is essential for the polymerization, the preorganization of the monomers into fibrous nanostructures is a prerequisite for obtaining well-designed PDA.
Recently, diphenyldiacetylene (DPDA) has become a fascinating research focus. Compared with routine alkyl diacetylene, DPDA has an extended π-conjugated system and possesses a larger intermolecular stacking tendency, and this structure may exhibit better electrochemical properties and conductivity. However, the phenyl group in turn increases the steric hindrance for the arrangement. Hence more efficient strategies in assembly are required. In this review paper, we summarized different ways in the topochemical polymerization of DPDA and introduced some conducting PDPDA-based materials as photocatalyst. Through the elaborate design of side chains on the phenyl group of DPDA, or with an appropriate external template, it can be smoothly fabricated into the assembled structures with the formation of some intermolecular interactions for topopolymerization
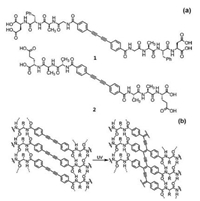
2. Self-assembled topochemical photopolymerization of DPDA 2.1. PDPDA-peptide nanostructuresThe uniformly aligned macroscopic assemblies of π-conjugated polymers would have great potential in electronic nanomaterials. It's worth mentioning that the Tovar group first reported watersoluble peptides embedded with diphenyldiacetylene to produce one-dimensional self-assembled alignment of conjugated polymers as intramolecular electronic conduits [18]. In this case, it is water manipulatable and would be able to undergo chromic alteration or gate-induced carrier formation upon polymerization. In the view of structure, the peptide side chain matters a lot for the polymerization, neither being too small to bury the diacetylene from water, nor being too large to avoid the precipitation of aggregates from solution (Fig. 1a). Besides, the intermolecular β-sheet hydrogen bonding acts as a control of local planarity and chirality of this assembly, which can be re-established under the regulation of pH.
|
Download:
|
| Fig. 1. (a) Molecular structures of DPDA diacids and peptides. (b) Stacked DPDA peptides and the resulting polymer. Adapted with permission [18]. Copyright 2012, ACS Publications. | |
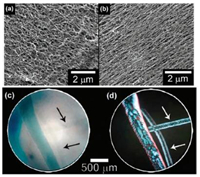
The oligopeptides with internal diacetylene can self-assembly into electronically delocalized amyloid-like nanomaterials, and then undergo topochemical polymerization into macrostructures under UV irradiation (Fig. 1b). To ensure the globally aligned polymerization of monomers, a macroscopic (cm scale) 'noodlelike' hydrogel (Figs. 2a and b) has been synthesized from a solution dispensing route invented by Stupp et al. [19]. Upon UV irradiation, the aligned peptide noodle will turn into 1D blue hydrogel noodles (Figs. 2c and d). This globally aligned peptide-PDA polymers can exhibit ambipolar semiconductor properties and may have application in future functional nanomaterials.
|
Download:
|
| Fig. 2. SEM images of polymerized gel of peptide 1 as (a) an unaligned hydrogel and as (b) an aligned peptide noodle. Peptide 1 hydrogel noodles before (colorless) and after (blue) polymerization as seen under a light microscope (c) without and (d) with crossed polarizers. Adapted with permission [18]. Copyright 2012, ACS Publications. | |
2.2. Dipeptide appended DPDA derivatives
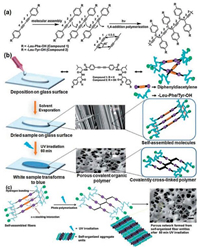
Porous covalent organic polymers (COPs) inspired from natural zeolite materials have become an important counterpart, as compared with Metal Organic Frameworks (MOFs) and Covalent Organic Frameworks (COFs), for gas adsorption due to environmental issues. A morphology-controlled solid state polymerization of dipeptide appended DPDA derivatives has been developed by Das and coworkers in 2017 for CO2 capture [20]. The peptide side chains on DPDA promote the molecular assembly through hydrogen bonding, resulting in an ordered alignment with a tilt angle of 45°. The distance between adjacent DPDA is as small as 4.9 Å. It can then easily undergo 1, 4-addition to get polymerized under UV irradiation. In view of the microstructure, the intermolecular π-π stacking interactions and hydrogen bonding between the amide group in their selfassembled state lead to the transform from fibrillar to porous network morphology after exposure to UV light. The UV light induced polymerization weakens the hydrogen bonding and changes the self-organization patterns. The above-stated mechanism of self-assembly, polymerization, and characterization are shown in Fig. 3. After polymerization, the surface area, porosity, pore volume, CO2 sorption capability and thermal stability are better than the corresponding monomers. This structure may be useful in design of adsorbents and catalysts for industrial applications.
|
Download:
|
| Fig. 3. (a) Typical topochemical polymerization mechanism. (b) Schematic representation of the preparation method used for compound 1 on a glass substrate for photochemical reaction in the solid state for 1 h. (c) Possible self-assembly mechanism of the dipeptide appended diphenyldiacetylene based compound 1. Adapted with permission [20]. Copyright 2017, The Royal Society of Chemistry. | |
2.3. Bis(imidazolyl) DPDA
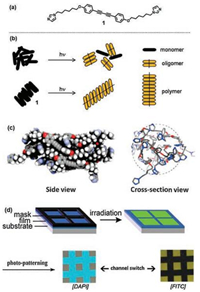
In 2015, Zhu and coworkers demonstrated a self-templated photopolymerization with bis(imidazolyl) diphenyldiacetylene. The highly uniform topochemical alignment attributes to the cylindrical self-assembly which strengthened the preorganization of the diacetylene moiety of monomers [21]. In particular, the imidazole moiety on both sides of DPDA ensures the self-assembly structure, while monoimidazolyl or nonimidazolyl DPDA can only form oligomer rather than polymer upon polymerization (Figs. 4a and b). To investigate the specificity of molecular structure from computational methods, molecular dynamics simulations are conducted to explain the self-aggregation pattern (Fig. 4c). Notably, the bilateral imidazole units, which have some hydrophilic properties and can stretch toward the water medium, guarantees the diacetylene moiety to stay at the middle of the cylinders and forms this self-sorted aggregation mode. On this basis, it is able to form high-molecular-weight polymers through the certain molecular structure. This self-assembled unimolecular photopolymerization has high polymerization degree for over 40 according to GPC result.
|
Download:
|
| Fig. 4. (a) Chemical structures of the compound 1. (b) Schematic representation of the unimolecular photopolymerization process in aqueous media. By UV irradiation under this solvent condition, 1 can form the corresponding polymer species. (c) Representative cylindrical self-assembly conformation of 1 from molecular dynamics simulations. (d) Imaging of the square areas before and after irradiation at 365 nm for 300 s, and the photo-patterning under DAPI mode and FITC mode. Adapted with permission [21]. Copyright 2015, ACS Publications. | |
Moreover, the above-mentioned material shows the aggregation-induced emission enhancement (AIEE) activity both in colloidal aqueous solution and in solid state thin films. The column-shaped nanostructure from AFM demonstrated that the peculiar self-assembly is responsible for the AIEE effect. A barrier was established to restrict the rotation of two phenyl rings in the self-assembly system, which explains for the enhanced photoluminescence in this structure. Besides, we can find a regionally selective imaging by switching of different detection modes. A mask was placed on the monomer film for region-selective photoirradiation. Either the square or the lane was luminescent from a FITC mode or DAPI mode, respectively (Fig. 4d)
3. External-templated topochemical photopolymerization of diphenyldiacetylene 3.1. Natural polysaccharide schizophyllan (SPZ) as a one-dimensional moldThe π-conjugated polymers have distinct advantages in stable conductivity. However sometimes, the simple polymerization may result in amorphous polymer-aggregates. Unlike the previously stated self-assembled structures, for monomers lack of distinct self-assembly characters, an external template can be employed to ensure the parallel alignment for the topochemical polymerization to prevent the polymer strands from being highly entangled
In 2005, Shinkai and coworkers published a Schizophyllan (SPG)-templated polymerization to induce the chiral-twisted packing of diphenyldiacetylene [22]. SPG is an extracellular β-1, 3-glucan repeated at every three units as shown in Fig. 5a. It has long been researched on for its solvent-induced reversible structure transition between a triple-stranded helical structure (t-SPG) in water and individual single-strands (s-SPG) in dimethyl sulfoxide [23]. Particularly, SPG has a one-dimensional tubular cavity at the center to accommodate hydrophobic guests (Fig. 5b). Thus, closely-packed monomers can pre-organize in SPG and form polymers with fibrous morphologies under UV or γ-ray irradiation. The host can undergo a renaturation process to turn from s-SPG to t-SPG for guest encapsulation (Fig. 5c). Unlike cyclodextrins, which have strict shape- and size-selectivity, SPG can fit the various structures and sizes of guests. This SPG-templated polymerization is not only suitable for several PDPDA, but may also be suitable to other functional polymers.

|
Download:
|
| Fig. 5. (a) Chemical and (b) spatial structure of schizophyllan having a triplestranded helical structure (t-SPG). (c) Schematic illustration of our concept to use SPG as a 1D host to accommodate DPDAs within the helical superstructure of SPG and construct poly(DPDAs)-nanofibers through UV/γ-ray irradiation. Adapted with permission [22]. Copyright 2005, The Royal Society of Chemistry. | |
3.2. Block copolymer templates
In 2014, Campos and coworkers employed poly(styrene-b-acrylic acid) (PSPAA) as a block copolymer template for the alignment and photopolymerization of DPDA [24]. In the photoluminescence spectra, the original peak corresponding to DPDA near 420 nm diminished, and another peak near 520 nm corresponding to PDPDA appeared upon UV irradiation. (Fig. 6c)

|
Download:
|
| Fig. 6. (a) Chemical structures of the block copolymer PS-b-PAA, the monomer IDA, and the corresponding polydiacetylene after the BCP templated photopolymerization of the supramolecular ensemble PS-b-(PAA-sg-IDA). (b) AFM topography images of PS-b-P(AA-sg-IDAR=0.2) for a solvent-annealed film. (c) Corresponding image of the solutions under a UV light. Adapted with permission [24]. Copyright 2014, ACS Publications. | |
This template system makes use of non-covalent interactions between the block copolymer and DPDA to template the topochemical polymerization (Fig. 6a). The reaction can be conducted both in solution and in thin film. The main noncovalent interaction in solution state is the hydrogen bonding between the imidazole on monomer and the acrylic acid on poly acrylic acid (PAA) backbone that promote the photopolymerization. In addition to the hydrogen bonding, the microphase segregation of block copolymers in thin film results in hierarchical self-assembly of monomers, which will enhance the molecular alignment through ordered domains and fasten the topochemical polymerization. It only takes 20 s to get polymerized in thin films, compared with 20 min in solution. The segregation process in solid state is induced by solvent vapor annealing, resulting in dotlike nanopattern topography (Fig. 6b). Photo-patterning experiments in micrometer length scale are conducted through shadow-mask photolithography, which can be useful in fabricating microarrays of photoluminescent materials (Fig. 7).

|
Download:
|
| Fig. 7. Photopatterning experiments. (a) Solvent-annealed thin films of P(S-co-BrS)-b-P(AA-sg-IDA) are aligned with a shadow mask and cross-linked with 254 nm. (b) After a rinse and reannealing step, hierarchical patterns are achieved, (c) as observed by confocal microscopy in the 510-530 nm channel. Scale bar is 500 μm. Adapted with permission [24]. Copyright 2014, ACS Publications. | |
3.3. Co-micelle nano-ensemble
The external control of the luminescent color conversion is promising in developing new-generation emissive materials. In particular, the tuning of multicolor luminescence concerning triplet state is quite challenging. Recently, Zhu and coworkers reported a simple co-micellar strategy, taking advantage of photocrosslinking of diphenyldiacetylene, in which the two emissive bands could lead to a controllable progressive luminescent color conversion [25]. Hydrogen bonding between imidazole and acrylic acid forms the co-micelle nanostructures and the long alkyl chains contributes to the aggregation inside the micelle core.
The trimeric oligodiacetylene and the dimeric one can be sequentially synthesized in the course of UV irradiation (Figs. 8a and b). The polymerization degree can be determined by matrixassisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry within the encapsulation of block copolymer. The progressive luminescent color changes from light-green to white and to purplish blue, indicating the formation of trimer, mixture and dimer (Fig. 8c). The formation of triplet state here is because of fast intersystem crossing (ISC). The long-life broadband emissions can be explained by delayed fluorescence (TADF) and phosphorescence (PP)

|
Download:
|
| Fig. 8. (a) Chemical structures of the block copolymer P, the DPDA monomer M and the photoconverted oligo (DPDA) of dimer D and trimer T. (b) A proposed process for the preparation route for the micellar ensemble P-M as well as the photoinduced luminescent color conversions of the co-micellar system: with an optimized encapsulation of the block copolymer, the trimeric oligodiacetylene will be preferentially formed in the ensemble first (P-T); continuous irradiation of the system further increases the content of the dimeric oligodiacetylene to generate P-DT and P-DT2. The luminescent color conversion originates from the change in the emissive proportion of the trimer and the dimer. (c) emission spectra upon 345 nm excitation of P-M in toluene at (1) initial state and after photoirradiation at 254 nm for (2) 5 min, (3) 15 min and (4) 30 min, and the photographs of the corresponding states 2, 3, and 4 under a UV light (λ = 365 nm). Adapted with permission [25]. Copyright 2016, The Royal Society of Chemistry. | |
3.4. Rotaxane-based host-guest nanostructure
As self-assembled monomers can appear to have ordered alignment in aqueous solution due to the intermolecular hydrogen bonding and π-π stacking within their distinct structure, external templates can in turn adjust the self-assembly conformations to some extent. In 2018, Zhu and coworkers reported a photocrosslinking of rotaxane-based host-guest nanostructure [26]. It is rare for host-guest complexes to form rotaxane-based nanostructures with photochemical reactions. Here, cucur [7] bituril (CB [7]) was employed as a host to bind with the cationic guestpositively charged viologen group on DPDA, with the bulky isophthalate as a stopper (Figs. 9a and b). Without the rotaxanebased host, this surfactant-like DPDA can undergo self-assembly and get polymerized into rodlike nanostructures (Figs. 9c and e). On the occurrence of CB [7], it presents irregular aggregates (Fig. 9d). This is because of the steric hindrance between the adjacent CB [7] rings that hampers the parallel stacking interaction. Upon UV irradiation, it forms a crown-shyness-shaped distinct interval with channel-like gaps, which is resulted from the unreacted circular aggregates that hydrophilic CB [7] rings stretch in aqueous solution and hydrophobic diacetylenes buried inside (Figs. 9f and g).

|
Download:
|
| Fig. 9. (a) Chemical structures and schematic illustration of compound 1 and the resulted product 2 after photo-cross-linking. (b) Schematic illustrations of 1 ⊂ CB[7] and its aggregation state as well as the corresponding [2]rotaxane-based nanoarchitecture 2 ⊂ CB[7] after photo-cross-linking. TEM images of monomers (c) 1 self-assembled into rodlikenanostructures, and (d) irregular 1 ⊂ CB[7] aggregates. TEM imageof (e) 2 showing rodlike morphology similar to its monomer self-assemblies, indicating the stacking mode was fixed during photo-cross-linking, and (f) 2 ⊂ CB[7] showing a crown-shyness-shaped morphology due to the threading of CB[7] along with channel-like gaps after photoirradiation. (g) Schematic representation of 2 ⊂ CB[7] assembled cross section. Adapted with permission [26]. Copyright 2018, ACS Publications. | |
4. Application of conducting polymer nanostructures in photocatalysis for organic pollutants degradation 4.1. Conducting polymer nanostructures for photocatalysis
As to the energy and environment issues, the semiconductor nanomaterials, especially the widely explored TiO2, can be applied in the photocatalytic conversion of solar energy. Traditional oxidebased semiconductors have limitations in low quantum yield and ultraviolet irradiation, due to the charge-carrier (e-/h+) recombination and the large bandgap, respectively. Although improvement made in doping and surface modification can extend the active absorption region towards the visible area, the modified photocatalytic activity is still not enough for large-scale production and commercial use [27, 28].
Recently, polymer-based nanofibers have been invented to become an equally competitive opponent for photocatalysis under visible light [29, 30]. Conjugated polymer nanostructures excel in the low cost, mechanical flexibility, high carrier mobility and electrochemical activity [31]. In 2015, Remita and coworkers developed the PDPDA nanostructures through photopolymerization, and they could act as a visible-light-responsive photocatalyst in organic pollutants degradation [32]. Being able to directly harvest light from solar energy, this stable and cheap semiconducting-based polymer would become promising in developing photocatalyst for environmental protection.
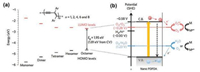
With use of the soft template approach, the PDPDA nanofiber was synthesized under UV irradiation in hexagonal mesophases consisted of oil-swollen surfactant tubes to ensure the fibrous network. Small-angle X-ray scattering spectra proves the hexagonal structure after polymerization (Figs. 10b-e). Surprisingly, it has better photocatalytic activity under visible light, compared with oxide-based semiconductors. Through cyclic voltammetry (CV) measurements and density functional theory (DFT) calculations, the LUMO-HOMO gap of PDPDA can be as small as 1.81 eV upon polymerization from both experimental and theoretical demonstration, indicating that excess electrons and holes will be formed once the photon energy exceeds the bandgap (E ≥ 1.81 eV or λ ≤ 685 nm). Investigated from the mechanism perspective, the migration of electrons and holes to the surface of semiconductor and the subsequent generation of highly oxidative radicals HO· are the key steps to degrade and mineralize organic pollutants. The mechanism is depicted as Fig. 11. Besides, no precious metal co-catalyst or sacrificial reagent is needed in this process. Experiments on the photocatalyzed degradation of methyl orange (MO) and phenol with PDPDA nanofibers for five times demonstrates that the high activity remains even after repeating cycles (Fig. 10a)

|
Download:
|
| Fig. 10. (a) Photocatalytic degradation of MO and phenol after up to five cycles measured after visible light irradiation of duration 240min. (b) Schematic of polymerization of DPDA by ultraviolet irradiation. (c) Small-angle X-ray scattering spectra of swollen hexagonal phases before (black squares) and after polymerization of 1, 4-DPDA by ultraviolet irradiation (red cycles). Inset: scheme of an oil-swollen hexagonal phase. (d) Absorption spectra of solid PDPDA nanostructures. Inset: Solid powder of PDPDA nanostructures. (e) Transmission electron micrograph of PDPDA nanostructures prepared by the soft templating approach. Adapted with permission [32]. Copyright 2015, Springer Nature. | |
|
Download:
|
| Fig. 11. Schematic representation of the photocatalytic mechanism and energy level calculation of polymer structures by density functional theory. (a) Energy diagram representing the evaluated HOMO and LUMO levels of PDPDA polymer. (b) Possible photocatalysis mechanism with charge separation in nano PDPDA, with electron reducing oxygen and hole oxidizing water; the holes and generated oxidative radicals can oxidize organic pollutants (noted as M), V.B. and C.B. represent the valence band and the conduction band of PDPDA polymer, respectively. Adapted with permission [32]. Copyright 2015, Springer Nature. | |
4.2. Conducting polymer nanofibers with ZnO nanoparticles (NPs) for solar light harvesting
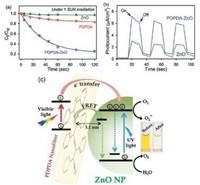
In solving problems regarding energy harvesting, the key factors for developing materials lie in the suitable photo absorption accords with solar spectrum, high performance charge separation to prevent the electron-hole recombination, and adequate energy to carry out a certain chemical reaction like photocatalysis [33]. The development of nanoheterojunctions can act as an efficient way for solar light harvesting. The incorporation of semiconductor nanoparticles into conjugated polymer nanofibers has shown great potential in solving the problems within traditional oxide-based semiconductors stated above. In 2015, Ghosh and coworkers reported a ZnO-doped PDPDA for solar light energy harvesting [34]. The adsorption of ZnO nanoparticles on polydiphenyldiacetylene nanofibers forms nanoheterojuctions, which is a high performance material for more efficient light harvesting. The ZnO NPs are generated on PDPDA nanofibers through a coprecipitation method.
Interfacial charge separation at the nanoheterojuction where electrons are transferred from PDPDA to ZnO NPs is the key step for light harvesting. Steady state and picosecond resolved fluorescence studies are conducted in order to precisely investigate this process. A Förster resonance energy transfer (FRET) is proposed from the surface defects of ZnO NPs (~5 nm) to PDPDA, which confirms the proximity with molecular resolution between PDPDA and ZnO. This structure enabled charge separation for about 5 fold increase in photocatalytic activity compared with polymer nanofibers counterparts in degradation of pollutants under sunlight (Fig. 12a). The photocurrent measurement serves as a direct evidence for high charge separation performance, as the photocurrent of PDPDA-ZnO is 2.5 times higher than pure ZnO (Fig. 12b)
|
Download:
|
| Fig. 12. (a) Photocatalytic degradation of MO in the presence of PDPB (red), ZnO (green) and PDPDA-ZnO (blue) under visible light (sun) irradiation. (b) Photocurrent responses of PDPDA-ZnO LHNH and ZnO NPs without any bias voltage under 1000 W/m2 incident power irradiation from a light source. (c) Schematic presentation of the interfacial carrier dynamics at the heterojunction showing the photocatalytic degradation of MO in aqueous solution. Adapted with permission [34]. Copyright 2015, Springer Nature. | |
The mechanism of this heterostructure for this photocatalytic degradation is integrated from the mechanism of the oxide-only or polymer-only nanostructures shown in Fig. 12c. The photoinduced electrons and holes escape from recombination and manage to migrate to the surface of the semiconductor, and consequently generate highly oxidative radicals (h+, HO· and O2·) to degrade organic pollutants
4.3. Conducting polymer with g-C3N4 for enhanced visible light photocatalytic activityIn 2017, Zhang and coworkers published a work on a new polymer composite of PDPDA and g-C3N4 [35]. Similar to the ZnO-compounded polymer, it also acts as an effective semiconductor photocatalyst under visible light irradiation to degrade organic pollutants, presenting higher activity than pure polymer or pure g-C3N4 caused by increased charge separation. Such an effect is attributed to the combination of the two components (Figs. 13a and b). The composites catalysts have good performance in stability even after five successive runs (Fig. 13c).

|
Download:
|
| Fig. 13. (a) Photocatalytic degradation of phenol over different catalysts under visible light irradiation. (b) Transient photocurrent response for PDPB, g-C3N4 and 50:2 CN–PDPDA photocatalysts. (c) Effect of cycling runs on RhB degradation in the presence of 50:2 CN–PDPDA under visible-light irradiation. Adapted with permission [35]. Copyright 2017, The Royal Society of Chemistry. | |
4.4. Solid state synthesis of conjugated polymer with carbon dots for enhanced light absorption and conversion
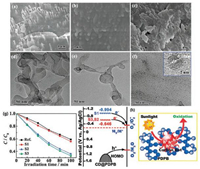
In 2017, Chang and coworkers first reported a green and simple method for the synthesis of carbon-dot-incorporated PDPDA nanohybrids [36]. In particular, the synthesis route is distinct from previous reported works without requiring a template, solvent or surfactant. The monomer was added to a Teflon-lined autoclave and heated at 200 ℃ for 50 min. The nanostructures transformed from nanothorn to spherical particles, and the number of carbon dots increased with the rise of temperature (Figs. 14a-f). Carbon dots act as a promising zerodimensional carbon material possessing semiconductor-like properties [37]. The incorporation of carbon dots into PDPDA can not only dramatically increase the light absorption, but also increase the photocatalytic performances with great performance in solar energy utilization. The experiment of the degradation on MO under visible light indicates that 67% degradation rate is achieved after 100 min, compared with the 42% for PDPDA only (Fig. 14g). The energy level diagram and the photoinduced charge migration mechanism is depicted in Fig. 14h.
|
Download:
|
| Fig. 14. (a–c) SEM images of S1–S3, the synthetic temperature of which is 100 ℃, 150 ℃, and 200 ℃, respectively; (d–f) TEM images of S1–S3, respectively; the inset in (f) shows the HRTEM image of one nanoparticle. (g) Photocatalytic degradation of MO in the presence of samples Ref. and S1–S3. (h) Schematic energy level diagrams of S1–S3 and possible processes of photoinduced charge migration. Adapted with permission [36]. Copyright 2017, The Royal Society of Chemistry. | |
5. Conclusion
We articulated the self-assembled and external-templated ways to achieve the preorganization of DPDA monomers. The organized alignment ensures the anisotropic properties upon polymerization and can perform better structure-specific characteristics. With extended π-conjugated system, PDPDA have distinct electrical and optical properties, which are promising in developing optoelectronic functional devices. We then introduced several conducting polymers based on the PDPDA structure as a photocatalyst for organic pollutants degradation. This platform exhibits better catalytic activity than traditionally developed oxide-based semiconductors in solar energy harvesting. The low cost, high electrochemical activity and mechanical flexibility all signify PDPDA as a potential intelligent material for future energy and environmental issues. Future work may focus on other templates for the preorganized alignment and extend the monomer scope to various substituents to explore better optoelectronic properties. The different polymeric structures and polymerization degrees may exhibit distinct π-conjugation characteristics. Additionally, progress can also be made in the doping or surface modification of PDPDA, to further improve the visible light absorption and energy conversion. The increase in photocatalytic activity can in turn ensure the better utilization of solar energy and more efficient degradation of organic pollutants
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (NSFC, No. 21644005), National Program for Thousand Young Talents of China, and State Key Project of Research and Development (No. 2016YFC1100300).
| [1] |
L. Zhu, Y. Zhao, J. Mater. Chem. C 1 (2013) 1059-1065. DOI:10.1039/C2TC00593J |
| [2] |
C. Hang, H.W. Wu, L.L. Zhu, Chin. Chem. Lett. 27 (2016) 1155-1165. DOI:10.1016/j.cclet.2016.04.003 |
| [3] |
H. Wua, B. Wu, X. Yu, P. Zhao, W. Chen, L. Zhu, Chin. Chem. Lett. 28 (2017) 2151-2154. DOI:10.1016/j.cclet.2017.08.002 |
| [4] |
L.H. Pang, J.M. Li, X.M. Lu, Q.H. Lu, Chin. Chem. Lett. 28 (2017) 1358-1364. DOI:10.1016/j.cclet.2017.04.009 |
| [5] |
Q. Qi, T.X. Zhao, B.L. An, X.Y. Liu, C. Zhong, Chin. Chem. Lett. 28 (2017) 1062-1068. DOI:10.1016/j.cclet.2016.12.008 |
| [6] |
H.L. Dai, Y.F. Geng, Q.D. Zeng, C. Wang, Chin. Chem. Lett. 28 (2017) 729-737. DOI:10.1016/j.cclet.2016.09.018 |
| [7] |
J. Tan, W.J. Chen, J. Guo, Chin. Chem. Lett. 27 (2016) 1405-1411. DOI:10.1016/j.cclet.2016.06.050 |
| [8] |
Z.Y. Zhang, T. Li, Chin. Chem. Lett. 27 (2016) 1209-1222. DOI:10.1016/j.cclet.2016.05.031 |
| [9] |
J.H. Kim, E. Lee, Y.H. Jeong, W.D. Jang, Chem. Mater. 24 (2012) 2356-2363. DOI:10.1021/cm300762j |
| [10] |
X. Chen, S. Kang, M.J. Kim, et al., Angew. Chem. Int. Ed. 49 (2010) 1422-1425. DOI:10.1002/anie.200905041 |
| [11] |
B. Yoon, S. Lee, J.M. Kim, Chem. Soc. Rev. 38 (2009) 1958-1968. DOI:10.1039/b819539k |
| [12] |
D. Chen, L. Tang, J. Li, Chem. Soc. Rev. 39 (2010) 3157-3180. DOI:10.1039/b923596e |
| [13] |
W.L. Xu, M.D. Smith, J.A. Krause, et al., Cryst. Growth Des. 14 (2014) 993-1002. DOI:10.1021/cg401380a |
| [14] |
G. Wegner, Z. Naturforsch. Teil B 24 (1969) 824-832. DOI:10.1515/znb-1969-0708 |
| [15] |
M.A. Reppy, B.A. Pindzola, Chem. Commun. (2007) 4317-4338. |
| [16] |
D.J. Ahn, J.M. Kim, Acc. Chem. Res. 41 (2008) 805-816. DOI:10.1021/ar7002489 |
| [17] |
H. Peng, J. Tang, J. Pang, et al., J. Am. Chem. Soc. 127 (2005) 12782-12783. DOI:10.1021/ja053966p |
| [18] |
S.R. Diegelmann, N. Hartman, N. Markovic, J.D. Tovar, J. Am. Chem. Soc. 134 (2012) 2028-2031. DOI:10.1021/ja211539j |
| [19] |
S. Zhang, M.A. Greenfield, A. Mata, et al., Nat. Mater. 9 (2010) 594. DOI:10.1038/nmat2778 |
| [20] |
S. Bhowmik, M. Konda, A.K. Das, RSC Adv. 7 (2017) 47695-47703. DOI:10.1039/C7RA09538D |
| [21] |
L. Zhu, X. Li, S.N. Sanders, H. Ågren, Macromolecules 48 (2015) 5099-5105. DOI:10.1021/acs.macromol.5b01488 |
| [22] |
T. Hasegawa, S. Haraguchi, M. Numata, et al., Org. Biomol. Chem. 3 (2005) 4321-4328. |
| [23] |
T. Yanaki, T. Norisuye, H. Fujita, Macromolecules 13 (1980) 1462-1466. DOI:10.1021/ma60078a019 |
| [24] |
L. Zhu, H. Tran, F.L. Beyer, et al., Chem. Soc. 136 (2014) 13381-13387. DOI:10.1021/ja507318u |
| [25] |
L. Zhu, M.T. Trinh, L. Yin, Z. Zhang, J. Am. Chem. Soc. 7 (2016) 2058-2065. |
| [26] |
M. Zhu, L. Yin, Y. Zhou, H. Wu, L. Zhu, Macromolecules 51 (2018) 746-754. DOI:10.1021/acs.macromol.7b02736 |
| [27] |
S. Linic, P. Christopher, D.B. Ingram, Nat. Mater. 10 (2011) 911-921. DOI:10.1038/nmat3151 |
| [28] |
E. Grabowska, A. Zaleska, S. Sorgues, et al., J. Phys. Chem. C 117 (2013) 1955-1962. DOI:10.1021/jp3112183 |
| [29] |
Q. Luo, L. Bao, D. Wang, X. Li, J. An, J. Phys. Chem. C 116 (2012) 25806-25815. DOI:10.1021/jp308150j |
| [30] |
M. Zhang, W.D. Rouch, R.D. McCulla, Eur. J. Org. Chem. 2012 (2012) 6187-6196. DOI:10.1002/ejoc.201200437 |
| [31] |
Y.Z. Long, M.M. Li, C. Gu, et al., Prog. Polym. Sci. 36 (2011) 1415-1442. DOI:10.1016/j.progpolymsci.2011.04.001 |
| [32] |
S. Ghosh, N.A. Kouamé, L. Ramos, et al., Nature Mater. 14 (2015) 505-511. DOI:10.1038/nmat4220 |
| [33] |
N. Serpone, A.V. Emeline, J. Phys. Chem. Lett. 3 (2012) 673-677. DOI:10.1021/jz300071j |
| [34] |
S. Sardar, P. Kar, H. Remita, et al., Sci. Rep. 5 (2015) 17313. DOI:10.1038/srep17313 |
| [35] |
J. Lei, F. Liu, L. Wang, Y. Liu, J. Zhang, RSC Adv. 7 (2017) 27377-27383. DOI:10.1039/C7RA03534A |
| [36] |
S. Hu, Y. Zhou, C. Xue, J. Yang, Q. Chang, Chem. Commun. 53 (2017) 9426-9429. DOI:10.1039/C7CC05526A |
| [37] |
S. Hu, Chem. Rec. 16 (2016) 219-230. DOI:10.1111/tcr.201500225 |
 2018, Vol. 29
2018, Vol. 29 


