b College of Food Science and Engineering, Ocean University of China, Qingdao 266003, China
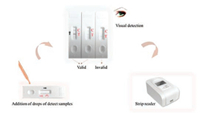
Lateral flow assay (LFA) is chromatography-based simplified sensor format for rapid, on-site detection of ligands. In recent years, strip biosensors have attracted considerable interest due to their portability, rapid assay time, cost-effectiveness and ease of use [1]. As a disposable sensor built in a user-friendly device, the strip detection platform offers possibility to perform on-site visual assays, which can be easily interpreted by untrained personnel. The results of such qualitative tests can be visually assessed by observing the intensity of the colored band, while quantitative data can be obtained by recording the colorimetric responses with a hand-held strip reader [2-5], which are based on the chargecoupled device (CCD) system or PMT (photomultiplier tube) with excellent sensitivity based on accurate counting of emitted photons.
Strip biosensors are cost-effective and can be used as point-ofcare tools in medical, food, diagnostics and environmental areas. Usually, quantitative or qualitative detection is based on the application of liquid samples on prefabricated nitrocellulose strips containing dry reagents that activate a signaling mechanism, typically colorimetric or fluorometric (Fig. 1) [6, 7].
|
Download:
|
| Fig. 1. Quantitative and qualitative analysis platform for the LFA systems. | |
Different recognition elements are used for the development of strip biosensors, such as aptamers and antibodies. Being short single-stranded DNA or RNA (usually DNA) that can bind to target analytes with a three-dimensional conformation of high specificity and affinity [8, 9], aptamers created through systematic evolution of ligands by exponential enrichment (SELEX) [10] from a combinatorial DNA library, are another example of easily recognizable elements. The length of an aptamer varies between 10 to 100 nucleotide bases normally, and the typical structural motifs include stems, inner loops, purine-rich bulges, hairpin structures, kissing complexes, pseudoknots and G-quadruplex structures. Aptamers can bind to multiple targets after experiencing adaptive conformational changes and three-dimensional folding [11], with high affinity and specificity. Nowadays, based on structural modifications of nucleic acid compositions, aptamer biosensors had been widely applied to various analytical fields as exemplified by the applications in food safety and medicine [12, 13].
Another recognition element, antibodies, are immunoglobulins that can specifically bind to a corresponding antigen [14]. Antibody production occurs in response to foreign antigen secretion, in both humans and animals. Antigen secretion by foreign objects, such as bacteria and viruses, leads to the recruitment of a variety of immune cells, and the subsequent B lymphocytes-antigen interaction, which results in B cell proliferation, differentiation, and antibody production. Laboratorial antibody production begins with immunization of experimental animals, such as rats, rabbits, mice, sheep, horses, goats and chickens [15].
2. Comparison of aptamers and antibodiesThough antibodies are widely used in analytical methods development in food inspection, diagnostics [16, 17], they are not ubiquitously applicable, because of their disadvantages. The main argument against their use is that antibodies are produced in vivo, involved with immunogenicity limitation, and with high potential of batch difference. Another problem is that antibodies are easily denatured and cannot be renatured, which limits the chemical modification and development of new analytical methods [18-21].
Compared with the antibodies, aptamers show significant advantages in terms of the easy generation, wide target range, easy modification, and so on [18, 22, 23]. Thus, aptamers appear to be superior alternatives to antibodies. And, as the aptamer-based biological researches are still in initial stage, the practical applications of aptamers in detection and diagnostics are still lagging behind those of antibody-based tests [21, 24].
2.1. Aptamers are more flexible than antibodies but the stability needs improvementFor bioanalytic applications, aptamers are suitable alternatives for nearly all antibody-based designs, in which both aptamers and antibodies can be used as molecular recognition elements [21], and aptamer-based biosensors have numerous merits, such as high sensitivity, low limit of detection (LOD), and excellent selectivity and stability. For example, a sensitive fluorescence anisotropy method for detection of mercury (ll) has been developed using a thymine-Hg2+-thymine complex structure [25]. A label-free aptamer-based sensor was developed, in which aptamers are immobilized on a support and target-binding signals are detected using fluorescence. Hg2+ ions present in an aqueous solution become sandwiched between the hairpin-shaped double-stranded DNA due to the formation of the thymine-Hg2+-thymine complex, which holds the Hg2+ ions in proximity to the surface of GO (graphene oxide) sheets. As a result, the fluorescence emission of GO is quenched. Another application is a fluorescent biosensor for human immunodeficiency virus Tat (HIV Tat) protein detection, which was based on the complementary of the two singlestranded oligonucleotides leading to the change of fluorescent signals [26, 27]. In order to enhance transducer signals, a colorimetric aptasensor based on aptamer-aptamer linkage was designed for multi-target detection, and the extension of aptamer DNA sequences significantly enhanced analytical sensitivity of this aptasensor in sensing applications for two small molecule targets [28]. Similarly, an aptamer-based colorimetric detection of vascular endothelial growth factor (VEGF) using a branched DNA cascade amplification strategy was recently developed. With this method, when the target protein-VEGF is detected in the sample solution, the aptamer structure forms a bio-functional hairpin probe (HP) [29, 30].
In spite of structural flexibility, the aptamer might be unstable in some conditions, as single-stranded nucleic acids are easy to be self-assembled or could entangle with each other. For this reason, in order to improve the sensitivity of the "strip sensor", it is necessary to optimize the recognition condition to avoid or minimize occurrence of the nucleic acids self-folding when using the aptamer as a bio-recognition element in the lateral flow strip sensor. So, the practical application of aptamer-based sensing and diagnostics is still lagging behind those of antibody-based tests [19].
2.2. Aptamer are more accessible than antibodies for nonimmunogenic and toxic targetsNon-immunogenic and toxic targets are not conducive to antibody generation, but are applicable in aptamer generation [31]. For example, malachite green (MG) is a fungicide approved for use in aquarium fish, but has potential health risk to humans [19]. Traditional detection methods for MG such as high-performance liquid chromatography (HPLC) or liquid chromatography-mass spectrometry (LC-MS), are time-consuming and expensive. It was difficult to develop rapid screening methods for MG detection, until a MG aptamer was developed in 1999. Grate et al. developed a 38-mer RNA aptamer that bound to MG with a dissociation constant (Kd) at less than 1 μmol/L [32], and a fluorescence-based screening assay for MG detection was developed by Stead et al. [33]. Other non-immunogenic targets like such as Apple StemPitting Virus (ASPV) and organophosphorus pesticide, are also paired with specific aptamers. A label-free aptamer-based sensor for ASPV detection was proposed by Lautner and coworkers [34]. A novel sensor for an organophosphorus pesticide namely Malathion was developed using PDDA (polyelectrolyte polydiallyldimethylammonium chloride) associated with the aptamer, and AuNPs (gold nanoparticles) were used for the signal transduction [35].
2.3. Aptamers are more applicable for biochemical separationAffinity and specificity are two key factors for biochemical separation. Since the aptamers bound to the small molecule targets with a Kd at less than 1 μmol/L in most cases, the separation between the aptamer-targets complex is inherently easier than antibodies-targets. Release of targets from the aptamer-target complex usually depends on rising temperature or adjusting pH, which would denature the antibodies. An aptamer-based microfluidic device for thermally controlled affinity extraction was designed with solid phase extraction (SPE) operation for extraction and purification of adenosine monophosphate (AMP) at physiologically relevant concentrations [36, 37]. Proteins were also extracted using aptamer-based designed methods. A DNA aptamer specific for Thermus aquaticus DNA polymerase (Taq-polymerase) was immobilized on magnetic beads, which were prepared in the purification of recombinant thermus [38]. Similarly, aptamerbased affinity purification for His-tagged proteins was developed. Aptamer-modified magnetic beads were successfully applied for purification of different His-tagged proteins from complex E. coli cell lysates. Imidazole (1 mol/L in PBST) was used to competitively displace the His-tag of the protein [39]. Another example was for the MutS protein purification, in which the protein was eluted by 6 mol/L urea [40]. Similarly, Adam et al. released and eluted the bound protein using 20 mmol/L Tris buffer containing 8 mol/L urea, pH 7.3, at 50 ℃ [41].
3. The principle of lateral flow strip and its main formats 3.1. The analytical merits of LFABiomolecules possess excellent properties as building blocks of analytical sensors, such as naturally availability in quantities, good biocompatibility, structurally diversity, optical chirality, multiple possible coordination modes, and prices amenability [42]. However, developing a rapid and affordable biosensor for detecting biomolecules remains a significant challenge, because of the interference in samples and difficulties in antibody generation, and the false positive results. Nucleic acid-based techniques such as DNA hybridization, polymerase chain reaction (PCR) and DNA microarrays, can achieve high specificity; however, these methods often require expensive instruments and professional skills, which limit their practicability in point-to-care (POC) diagnostics under extremely resource-limited environments [42]. LFAs are fabricated with strip, recognition elements, and visible results, and can be used for qualitative, semi-quantitative, and quantitative analysis in resource-limited or non-laboratory environments [21]. Nowadays, LFA is the most widely spread immunochromatographic system which is an assembly of several plain porous carriers impregnated with immunoreagents [43].
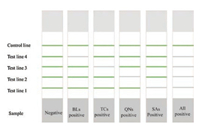
3.2. Main components of the stripAs shown in Fig. 2, a typical strip is assembled by five parts: the sample pad, conjugate pad, nitrocellulose membrane, absorbent pad, and backing plate. A liquid sample flows along the carriers, detectable complexes are formed in certain zones of the test strip (Test line), and thus visible results were collected. Control line was designed for the validation of the strip.

|
Download:
|
| Fig. 2. The typical lateral flow test strips configuration (A) and the result judgment of detection (B, C). Reproduced with permission [52]. Copyright 2002, Elsevier Advanced Technology. | |
Sample pad: Function of the sample pad is to transport the sample to the bottom components of the strip. It is made of cellulose and/or glass fiber. Sample pads are sometimes designed to pretreat the samples, such as separation of sample components, removal of interferences, adjustment of pH, etc. [44]. The sample pad could be socked in the buffer (pH 8.0) contains 0.25% Triton X- 100, 0.05 mol/L Tris-HCl, and 0.15 μmol/L NaCl [45]. An aptamerbased strip biosensor for detection of ATP was developed recently, and the sample pad was saturated with TBS buffer (20 mmol/L TrisHCl, 140 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L CaCl2, 1 mmol/L MgCl2, pH 7.2) [46]. The latest strip biosensor for detection of ochratoxin A showed that the sample pad was previously saturated with PBS solution containing 1% OVA and dried at 37 ℃, and stored under dry conditions [47].
Conjugate pad: It is the second pad where the bio-recognition molecules were loaded in the strip. The materials of the pad are generally glass fiber, cellulose, polyesters and other materials which were able to immediately release the labeled conjugate upon contact with moving liquid sample [44]. The conjugate pad should be pretreated before use as well. Sometimes, poor preparation of the labeled conjugate can influence the sensitivity of test strip. There is an aptamer-based lateral flow strip for on-site detection which the conjugate pad was pretreated with PBS solution containing 1% OVA, 0.25% Tween 20, 2% sucrose, 0.02% NaN3 and dried at 65 ℃ [47].
Nitrocellulose (NC) membrane: It is the largest pad in the strip and affects importantly the sensitivity of the strip. Test and control lines are usually lineated on the nitrocellulose membrane. The function of the membrane is to provide support and good binding to capture probes, while the sample and the detection conjugate are directed over the membrane to the reaction zone. To achieve this goal, the nitrocellulose membrane must have a uniform high adsorption capacity and also need to have a certain porosity and humidity to ensure the capillary flow of aqueous samples. Streptavidin (SA) is widely used as the connector combining the biotinylated probe with the membrane, in order to load the probe in the test zone. For example, a streptavidin-DNA probe 1 and streptavidin-DNA probe 2 conjugates were normally dispersed on the NC membrane as test line and control line, respectively [48]. On the contrary, for rapid detection of ochratoxin A (OTA) with lateral flow immunoassay biosensor, a BSA conjugate of OTA and a goat anti-rabbit lgG diluted in PBS were applied to the NC membrane to form the test and control line, respectively [49].
Adsorbent pad: It is the last pad in the strip. It functions just like a blotting paper, maintaining flow rate of the liquid over the membrane and stopping back flow of the sample [44].
Backing plate: It is also known as backup plate. All beforementioned pads are fixed in a backing plate. It is used for supporting for strip assembling and result reading.
All these pads are often laminated 2 mm with each other in sequence in order to ensure the liquid solution migrating through the LFA strip [50].
3.3. Different immunochromatographic systemsSeveral typical formats, such as "sandwich" format, competitive format, and multiplex detection format, are designed in the LFAs for various biomolecule detection.
Sandwich detection is a classic format in LFA strip for detecting analytes which have multiple bonding sites. An AuNPs-based immunochromatographic assay for detection of ricin was developed which was based on the sandwich format (Fig. 3). Two highaffinity anti-ricin monoclonal antibodies were used. The anti-ricin B chain Mab was immobilized to a defined test zone on the NC membrane, while the anti-ricin A chain Mab was conjugated to AuNPs which served as a detection regent [51]. Similarly, an antiabrin-A monoclonal and polyclonal antibodies to develop a sensitive sandwich immunochromatographic assay, the LOD is 0.1 ng/mL [52]. There is also an application of DNA aptamers to the lateral flow test strips for detection of foodborne pathogens which used Qdots as the signal transduction element. This study provides proof-of-principle for highly sensitive aptamer-Qdot LF strip assays for rapid foodborne pathogen detection [7]. In this sandwich format, when the target molecule was added into the sample pad, color change on the detection zone can be observed. Recently, a new lateral flow strip assay built based on sandwich format for sensitive detection of vaspin was also published [53]. The new sandwich-type format in LFA was developed based on biotin-labeled V1 aptamer as a capturing probe and V49 aptamer conjugated with gold nanoparticles (AuNPs) as a signaling probe. A novel three-dimensional AuNPs-DNA network structure amplification strategy was employed to design a lateral flow biosensor in another paper. This method could improve the sensitivity by four orders of magnitudes compared to the traditional sandwich assays labeled with AuNPs [54].

|
Download:
|
| Fig. 3. Schematic description of the principle for the strip sensor with sandwich format. Reproduced with permission [51]. Copyright 2002, Elsevier. | |
Another mode is competitive format. No matter the recognition elements are aptamers or antibodies, there is competitive relationship between the targets and capture molecules. Two sensitive immunochromatographic assay using antibody probe for rapid detection of pharmaceutical indomethacin and brevetoxins have developed [55, 56]. The assay for the detection of brevetoxins was based on a competitive format using two antibodies. The primary antibody was conjugated with AuNPs (color reagent), and the secondary antibody (capture reagent) was immobilized within a defined detection zone (control line) on a diagnostic cellulose nitrate membrane. The toxin in sample compete with immobilized toxin in test line to bind with AuNPs conjugated Mab. The mobile AuNPs-monoclonal-toxin complex can be captured by the secondary antibody and the color changed [55]. In addition, competitive lateral flow immunoassay based on AuNPs for rapid qualitative and semi-quantitative analysis of ustiloxins A and B in rice samples was also published recently [57]. There are series of assays using aptamers probe for rapid detection in the past few years. A new strip biosensor has been developed based on well-distributed thrombin aptamer- functionalized AuNPs aggregates, which will arouse a catalytic amplification when the target recognizes its corresponding aptamer. This is an aptamer-cleavage reaction with enzyme catalytic amplification system, the LOD of which is 4.9 pmol/L under optimal conditions [58]. A LFA biosensor for protein analysis with aptamer-functionalized AuNPs as probes was performed by Hui Xu et al. [45]. At the same time, they compared the aptamer-based dry-reagent strip biosensor with the antibodybased dry-reagent strip biosensor, the results indicate that aptamer-based dry-reagent strip biosensor shows similar sensitivity and even superior with respect to specificity [45]. Another strip sensor developed on AuNPs and aptamer for sensitive detection of OTA was also published several years ago which was based on the competition for the aptamer between OTA and DNA probes (Fig. 4) [48].

|
Download:
|
| Fig. 4. Schematic diagram of the principle for the strip sensor with competition format. Reproduced with permission [48]. Copyright 2011, Elsevier Advanced Technology | |
4. The experimental elements for LFA
In the strip test, the most important three experimental parameters are bio-recognition molecules, the signal transduction elements, and the detection targets.
4.1. The bio-recognition molecules 4.1.1. AntibodiesBecause of the specific ability with the targets, antibodies are initially used as bio-recognition molecules in the test strip detection method. The antibiotic is one of the big families of the detection targets using antibody-based LFAs [59, 60]. An ultrasensitive LFA for non-pretreatment monitoring of chloramphenicol in raw milk was developed in 2015 [61]. The detection process was carried out in 8 min with a visual LOD of 0.3 ng/mL for qualitative detection and a LOD of 0.064 ng/mL for semi-quantitative detection. A new lateral flow immunoassay for rapid detection of ochratoxin A in wine and grape was performed in 2012 [49], which is the first assay capable of measuring the toxin in wine and grape must. In a typical LFA for OTA detection, the antibodies were labeled with AuNPs and flowed across a membrane, onto which partner reagents (a protein conjugate of OTA and antispecies IgG antibodies) were coated in spatially. A LOD of 1 μg/L and IC50 of 3.2 μg/L is showed in this assay.
However, antibody generation is not feasible for non-immunogenic or toxic targets. Detection using antibody based lateral flow strip remains challenged nowadays.
4.1.2. AptamersFor bioanalysis applications, aptamers are suitable for nearly all antibody-based designs. Aptamer expresses great flexibility in different formats because of nucleic acids' structural diversity, and good adaptive folding ability for non-immunogenic and toxic targets. In 2014, an aptamer-based LFA for the rapid and facile detection of aflatoxin B1 has been developed [62]. Aptamer specific for aflatoxin B1 was labeled with fluorescent material cyanine 5 (cy5) for optical identification. After optimization, the LOD for the dipstick assay was 0.1 ng/mL. Another application is the clinical analysis for specific detection of thrombin. A highly sensitive lateral flow immunoassay method using AuNPs and a pair of aptamer probes has been developed. The assay does not need multiple incubation and washing steps any more [50]. Only very few assays reported using lateral flow strip test method for antibiotic detection and other substances that are not allowed to exist regulatory.
4.2. The signal transduction elementsOptical characteristics of labels (such as AuNPs), fluorescence of fluorescent materials (such as fluorescent nanoparticles), quantum dots (Qdots) were widely applied for signal transduction [7]. Recently, a paper shows the advantages of time-resolved fluorescent nanobeads compared with several labels. In this experiment, time-resolved fluorescent nanobeads, fluorescent sub-microspheres, Qdots, and AuNPs-based LFA were first systematically compared with each other for the quantitative detection of ractopamine in swine urine based on competitive format [63]. Another comparison of immunochromatographic assay based on fluorescent microsphere and Qdot sub-microbeads for quantitative detection of aflatoxin M1 in milk was developed [64]. The labeled signal transduction elements are a critical factor for improving the sensitivity of LFA.
4.2.1. AuNPs (Gold nanoparticles)AuNPs, with diameter at 1~100 nm, are widely used as the marker for rapid detection in the lateral flow strip assay, because of the visible characteristic and excellent chemical stability [50, 65]. A strip biosensor was established based on well-studied thrombin aptamer-linked AuNPs aggregates, which will undergo a cracking reaction when DNA aptamers recognizes thrombin [58]. Another strip biosensor has been developed based on the aptamer-AuNPs for ATP detecting. It is the first time that a strip biosensor was reported for ATP analysis by combining the high affinity and sensitivity of aptamers with the unique optical properties of AuNPs [46].
4.2.2. QdotsCompared to the test strip based on AuNPs, the lateral flow test strips with DNA aptamers and Qdots demonstrate higher sensitivity in the foodborne pathogens detection. A highly sensitive assay based on red-emitting Qdots (Qdot 655) with sandwich format was reported, and this aptamer-Qdot lateral flow strip test successfully provides proof-of-principle for rapid foodborne pathogen detection [7]. Another published a fast and sensitive immunoassay of avian influenza virus based on label-free quantum dot probe in a sandwich format LFA. The gold nanoparticle labels were captured in the test zone and further dissolved to release a large number of AuNPs ions as a signal transduction bridge detected by the Qdots-based fluorescence quenching method. This assay shows high sensitivity and selectivity which was 100-fold greater than the enzyme-linked immunosorbent assay (ELISA) [66].
4.2.3. Fluorophore silica nanoprobesSilica nanoparticles loaded with fluorescein could be used as a sensing probe for the detection of small molecules [67]. Recently, researchers find a new means to detect the small molecule ATP using lateral flow strips of aptamer-gated silica nanoparticles. The assay previously showed that a homogeneous solution of aptamergated silica nanoparticles loaded with fluorescein could be used as a sensing method for small molecules. When the target molecule is added to the assay solution, the conformational change of the gate molecule results in the opening of the silica nanopores and allows the release of the captured fluorescent molecule [6].
4.2.4. Magnetic nanoparticles (MNPs)In order to improve the stability and sensitivity of the LFA, new signal transduction elements-MNPs emerge. Nowadays, MNPs have widely used in different biomedical applications such as the separation between biotin and nucleic acid [68], bio-detection [69], magnetic resonance imaging (MRI) [70], bio-sensing [71] and drug delivery. In those early days, lots of assays used the super paramagnetic nanoparticles as labels in lateral flow immunoassay [72-75]. A group designed an experimental study to test whether the size and magnetite content of MNPs affect the performance of lateral flow immunoassay [76]. Results showed that the detection time mainly depends on the size of MNPs, while the signal intensity is closely related to the magnetite content of the MNPs. The magnetic signal intensity remains stable for a long time. So, the smaller MNPs content with higher magnetism is more preferable for building fast and highly sensitive quantitative LFA tags. A highly sensitive lateral low immunoassay based on MNPs for quantitative detection of carcinoembryonic antigen was developed in 2016. In order to amplify the signal, secondary MNPs (carboxyl group modified) were linked with detection MNPs through biotinstreptavidin interaction [77].
4.2.5. Latex beadsConsidering the relative higher cost of AuNPs, some researchers used common dyes, such as blue dye, as labels for protein detection on LFA platform [78]. For example, a quantitative lateral flow aptamer strip sensor based on blue dye doped latex beads (modified with carboxyl group) was proposed in 2013 [79]. Blue dye doped latex beads and aptamer conjugates were captured on the test line in the presence of target DNA, to form an obvious blue band. After optimization, the LOD for the strip assay was 3.75 fmol/L synthesized DNA in human plasma sample. Now, great efforts are committed to the research ofmultiplex detection systems using lateral flow strip assay as exemplified by Seoho Lee et al.in 2016 [80], they presented a novel assay scheme that uses two-color latex labels for rapid detection of acute febrile illnesses (AFIs).
4.3. The detection targets 4.3.1. Clinical indicatorsATP: Adenosine-5'-triphosphate (ATP) is a purine nucleotide present in all living cells with a molecular weight of 507.18 g/mol. It plays a critical role in the regulation of cellular metabolism and biochemical pathways in cell physiology [6, 46]. There are two fast, sensitive biosensors developed for ATP detection by using ATP aptamer-functionalized nano-materials as sensing elements in 2016. The principle of the assay is based on the competitive reaction between the test probe (complementary with part of the anti-ATP aptamer) and the target ATP to combine with anti-ATP aptamers. After optimization, the LOD of ATP in the simulated urine was 20 μmol/L [46]. After a few months, another assay was developed by lateral flow strips via aptamer-gated silica nanoprobes, with the strips developed in this assay, a linear range between 100 μmol/L and 2 mmol/L ATP was obtained, and the LOD was 69 μmol/L with the internationally harmonized IUPAC's 3σ strategy [6].
Thrombin: Thrombin, an allosteric serine protease, is a key enzyme in the blood coagulation and wound healing processes. In response to bleeding, a complex series of clotting-factor interactions leads to its conversion by thromboplastin to thrombin, which transforms fibrinogen in plasma into fibrin. Due to the clinical importance of thrombin, it would be tremendously valuable to establish a fast and reliable way for the detection of thrombin [23]. Different approaches based on aptamer can be used for thrombin detecting [45, 58]. In 2009, an aptamer-based LFA assay was developed for thrombin detection. The response of the biosensor is linear over a range of 5-100 nmol/L with an LOD of 2.5 nmol/L (S/N = 3). In 2013, there was a signal enhancement in a LFA based on antibody-dual gold nanoparticle conjugates (30 nm GNPs and 16 nm GNPs). The LFA developed in this study was applied to detect thrombin concentration in the range of 0.5-120 nmol/L with a LOD of 0.25 nmol/L [50]. In 2015, a new class of strip biosensors has been established based on thrombin aptamer-linked AuNPs aggregates, which will undergo a cracking reaction when the target recognizes its homologous aptamer. Under optimal conditions, the strip reader exhibited a linear relationship between the peak area and the concentration of thrombin in the range of 6.4 pmol/L-500 nmol/L with an LOD of 4.9 pmol/L [58].
Carcinoembryonic antigen (CEA): Since CEA is an important biomarker in cancer diagnosis, the detection of CEA is crucial for clinical analysis. An efficient, selective LFA based on MNPs for sensitive detection of CEA was performed. Sensitivity evaluation showed a broad detection range of 0.25-1000 ng/mL for CEA, and LOD of the aptamer-based LFA was 0.25 ng/mL, which is significantly decreased, compared with that of the traditional LFA [77].
Human chorionic gonadotropin (hCG): The first homemade LFA was performed in 1976 to detect human choronic gonadotropin (hCG) in urine for pregnancy test [81]. In 1990, Beggs et al. firstly used AuNPs-based LFA to detect hCG, and AuNPs then been developed as the most commonly used marker in lateral flow strip assay [82]. The principle of this test is based on antibody-antigen specific interaction. Recently, a lateral flow immunoassay with an enhanced sensitivity and thermostability for detection of hCG was developed by using Pt nanoparticles with a peroxidase activity [83]. The sensitivity of the novel assay was determined to be improved by as much as 1000-fold compared to the conventional rapid test based on colored AuNPs.
4.3.2. BiotoxinOchratoxin: Some reviews introduced various lateral flow strip methods to the detecting of OTA, immunochemical methods with different formats, although not exhaustive, were developed recently [7, 84-88]. Retrospectively, LFA for OTA detection was firstly reported in 2005 and a LOD of 500 ng/mL was acquired [89]. Recently, there are several fast, sensitive biosensor strategies for OTA detection [47-49, 90-92], improving the visual LOD to be 1 ng/mL, and the quantitative LOD to be 0.18 ng/mL [48] (Table 1) [47-49, 89-92].
|
|
Table 1 LODs of different LFAs developed for ochratoxin A. |
Aflatoxin B1: A LFA based on aflatoxin aptamer for the rapid and simple detection of aflatoxin B1 (AFB1) has also been described [62]. The LFA format was based on a competitive reaction with biotin-modified AFB1 aptamer between target and cy5-modified DNA probes. After optimization, the LOD for the LFA was 0.1 ng/mL AFB1 in buffer and 0.3 ng/g AFB1 in corn samples, respectively.
Ricin: Ricin is a potent protein toxin isolated from the seeds of the castor bean Ricinus communis. A rapid and sample immunochromatographic assay based on Ricin antibody has been published [51]. In order to improve the sensitivity of the test strip, they used silver enhancement reagent to amplify the signal of AuNPs. This assay was reported and LOD of 50 ng/mL was achieved, after optimization, the assay sensitivity can be increased by silver enhancement to 100 pg/mL.
Shellfish toxins: Shellfish toxins are guanidine compounds that causes paralytic shellfish poisoning (PSP). Clinical symptoms including nausea, vomiting, diarrhea, abdominal pain, and respiratory distress can appear several minutes to several hours after ingestion [93, 94]. Several analytical methods (liquid chromatography with fluorescent detection, LC-MS/MS) have been developed and adopted for the monitoring of shellfish toxins in the past few years, and LFA is the simplest assay format with a satisfactory sensitivity in terms of detection time, equipment and operator expertise. A lateral flow strip assay based on AuNPs for detection of saxitoxin was developed in 2011, the LOD of the competitive format sensor was calculated to be 12 ng/mL and the whole detection process was finished within 35 min [95].
4.3.3. MicroorganismsEscherichia coli: Nowadays, there are only a few lateral flow immunochromatographic assays for detection of Escherichia coli. There is an application of aptamer and Qdots based-lateral flow test strips for detection of Escherichia coli [7]. A comparison is made between AuNPs and Qdots when they were utilized as the labeling material in the assay. The sensor produces a visible LOD of ca. 3, 000 live E. coli 8739 cells and 6, 000 live E. coli O157:H7 cells by visible colloidal gold detection while the LOD was enhanced ten-fold to be ca. 300-600 cells per LF test with Qdot 655.
Listeria monocytogenes: A few LFAs can be used to detect the Listeria monocytogenes. In 2014, a visual and sensitive AuNPs-based lateral flow detection of Listeria monocytogenes by sensing of RNA markers was developed [42]. When the aptamer migrated along the membrane by capillary action, the AuNPs accumulated at the designated area, which performed a visual test using sandwich format. Under optimized experimental conditions, 0.5 pg/mL L. monocytogenes could be detected. It could also be used to detect 20 CFU/mL L. monocytogenes from actual samples.
4.3.4. AntibioticsChloramphenicol: Although various sensitive strip tests are able to detect chloramphenicol, all LFAs of chloramphenicol were based on antibody but not nucleic acid aptamer (Table 2) [61, 96-98].
|
|
Table 2 LODs of different LFAs developed for chloramphenicol. |
Tetracyclines: Tetracycline and oxytetracycline (OTC), two typical antibiotics belonging to the tetracyclines family, was originally used for disease prevention. However, the abuse of tetracycline antibiotics, can result in drug residues in food. The tetracycline residue in animal-derived food has a potential risk of drug-resistant bacteria and other toxicity in humans. Efficient and fast supervision of tetracycline and OTC can greatly safeguard consumers' health. A LFA based on antibody and near-infrared (NIR) fluorescence materials was first developed for detection of tetracycline in milk. For qualitative analysis, the cut-off values of tetracycline were determined to be 4 ng/mL. In 2017, a new strip sensor for the detection of OTC in milk has been reported [59]. In this study, a competitive immunoassay format was established and nano-particles (GNP) were used as labeling material in LFA. Assay was validated by spiking OTC into antibiotic-free milk samples and tests could be accomplished with in 5 min without any other equipment. After optimizing experimental conditions, the visual LOD was 30 μg/L.
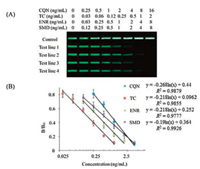
Others: There is a multiplex lateral flow immunoassay based on NIR (near-infrared) fluorescence for the simultaneous detection of four families' residual antibiotics (β-lactams, tetracyclines, quinolones and sulfonamides) in milk [60] (Fig. 5). In this study, broad-specific monoclonal antibodies were used as biorecognition molecules. Four competitive antigens were coated on different test positions of the nitrocellulose membrane as capture reagents. Four different mAbs and receptors were treated with NIR dyes as test reagents. If one of the antibiotics is present in the milk sample, the antibiotic residue will compete with the limited immobilized capture antigen for the NIR dye-antibody/receptor conjugate. After optimization, the LOD values for β-lactam, tetracycline, quinolone and sulfonamides were 8 ng/mL, 2 ng/mL, 4 ng/mL and 8 ng/mL, respectively (Fig. 6). The entire detection process can be completed within 20 min.
|
Download:
|
| Fig. 5. Schematic illustration of multiplex NIR-based LFA. Reproduced with permission [60]. Copyright 2016, Elsevier Advanced Technology. | |
|
Download:
|
| Fig. 6. (A) Typical photo image of NIR lateral flow strips for the detection of four different kinds of antibiotics using CQN, TC, ENR and SMD as reference analytes. (B) Calibration curves of the four antibiotics by plotting B/B0 against the logarithm concentration of analytes. Copied with permission [60], Copyright 2016, Elsevier Advanced Technology. | |
4.3.5. Metal ions
With the increasing motivation of detecting metal ions in chemical and biomedical fields, demand for highly sensitive and selective sensors is greater and greater. Many different ways to detect both diamagnetic and paramagnetic metal ions (Hg2+, Pb2+, Cr2+, Cu2+) have been developed [99]. These sensors are simple and highly sensitive (with LOD down to 2×10-4 ng/mL) (Table 3) [2-5, 100-103].
|
|
Table 3 Developed LFAs for detection of metal ions. |
4.3.6. Allergen
Food allergies have become an important health problem all over the world especially in industrialized countries. Food allergens includes eggs, gluten-containing cereals, fishes, peanuts, soybeans, milk, various nuts, crustaceans [104], etc. Lateral flow immunoassays for allergen detection in food are simplified versions of ELISA. The LFAs are cheap, fast, simple, and sensitive methods for on-site detection of allergens (Table 4) [73, 105, 106].
|
|
Table 4 Developed LFAs for detection of allergen. |
4.3.7. Pesticides
Due to the rising attention toward pesticide residues within food, water and soil in the world, and the demand for the development of fast, cost-effective, simple, highly sensitive and selective sensors has been increasing. A number of formats of immuno-analytical techniques for analyzing environmental pesticide residues are summarized in a review by C. Raman Suri (2009) [107], although not exhaustive. Lateral flow immunochromatographic test strip is more time-saving and cost-effective, earlier uses of LFA with hapten-protein as the bio-recognition molecules to detect pesticide residues were in 2003 [108] and 2007 [109], with the LODs were 0.5μg/L and 1.0 μg/L, respectively. Recently, a new LFA based monoclonal antibodies (mAbs) for the rapid and simple detection of 3-phenoxybenzoic acid (3-PBA) in river water has been described. In this study, anti 3-PBA monoclonal antibodies (mAbs) were developed by using PBA-bovine serumalbumin (BSA) as an immunogen which AuNPs as labels. After optimization, the cut-off value of the strips was 1 μg/mL 3-PBA, and the visual linear range was between 0 to 1 μg/mL [110].
4.3.8. Illegal adulterantsMelamine: Melamine (MA, 1, 3, 5-triazine-2, 4, 6-triamine) is an industrial chemical and has been widely used in the manufacturing of plastics, amino resins and flame retardants [111]. In 2007, investigations showed that MA was criminally added to milk, milk powder and other food products in order to increase the apparent protein content, which lead to the food safety concern [112]. There is an urgent need to explore a rapid and sensitive detection method to monitor the MA contamination of food, especially for on-site testing. As tabulated in Table 5 [112-115], LFAs offer many advantages when compared to other methods such as HPLC, liquid chromatography-mass spectrometry and gas chromatography/mass spectrometry [112, 113].
|
|
Table 5 Developed LFAs for detections of MA. |
Clenbuterol: Clenbuterol (CLE, 4-amino-α-[t-butylaminomethyl]-3, 5-dichlorobenzyl alcohol hydrochloride), a β 2-adrenergic agonists, can be used for treatment of pulmonary disease and asthma, enhance the growth rate of animals, and reduce fat accumulation, thus posing a human health risk [116]. Researches show that CLE can be accumulated in animal tissues and remained for long time, which will cause severe adverse effects on human and animal health such as cardiovascular and central nervous diseases [116-122]. Various methods have been developed for the detection of CLE residues, most of which are time-consuming and require tedious sample pretreatment and expensive equipment, limiting its practical use [122]. On the contrary, LFA is a wellestablished analytical method and widely used for on-site detection of CLE (Table 6) [121-123].
|
|
Table 6 Developed LFAs for detections of CLE. |
4.3.9. Genetically modified organisms
With the fast development of modern biotechnology and agricultural science, genetically modified organisms (GMOs) have been claimed as a complementary solution to enhance the crop productivity [31, 124]. But the concerns about their safety persist amongst the public, and the acceptance of these GMOs by consumers is still controversial. In some countries, GMOs cannot be imported or sold. Hence, qualitative and rapid on-site detection methods for GMOs were required [125]. GMOs contain an additional feature encoded by an introduced gene, which generally produce an exogenous protein. Hiroshi Akiyama et al. developed a lateral flow strip assay for the qualitative detection genetically modified rice which express CryIAc protein. The spiked recombinant CryIAc protein was successfully detected at the level of 0.012 mg/g in both unpolished and polished rice [125].
5. The optimization elements of the LFA 5.1. The molar ratio of bio-recognition elements to the signals transducersUndoubtedly, the molar ratio of bio-recognition elements to the transducers will affect the response of LFA and thus, the ratio needs to be investigated. In LFA sensor based on aptamers and AuNPs, too much aptamers would result in low sensitivity, and excessive AuNPs would cause higher background signal of the strip sensor. In order to increase the sensitivity of the strip sensor, the two factors were optimized for two sensitive OTA detection assays [48, 92]. Density of aptamers on the surface of AuNPs could influence the final color of the test and control lines [48]. The optimal ratio of aptamer to AuNPs was 1:2. Another antibody-based lateral flow strip assay for OTA was also developed to optimize the ratio of antibody-AuNPs conjugate and OTA, the optimal ratio is 2:1 [92]. After optimization, the LOD was 0.18 ng/mL and 1 ng/mL respectively. A technique for HBV detection was recently reported and a LOD of 0.1 nmol/L was achieved under optimized assay conditions [54]. Aptamer and AuNPs with five molar ratios were tested to reach a maximum efficiency. The signal increased with decreasing molar ratio from 3:1 to 1:1 and reached a maximum value at 1:1. In order to retain optimized assay conditions with high signal intensity but low background, a molar ratio of 1:1 was chosen.
5.2. The amount of the conjugates of bio-recognition elements and signals transducersA number of assays have optimized the factors, such as the amount of AuNPs-aptamer conjugates [2, 45, 47, 50, 100], the antibody-AuNPs nanoparticles conjugate dilutions [126], the concentrations of antibody-Qdots [97], and the amount of MWCNTs-DNA [3]. The AuNPs-aptamers were dispersed on a glass fiber membrane and then dried to form a conjugate pad of visible indicators [47]. The concentration of AuNPs-aptamer could straightforwardly affect the LOD in the aptamer-based lateral flow strip. Assay sensitivity was decreased with higher amounts of AuNPs-aptamer conjugate, and a final amount of 4-fold diluted AuNPs-aptamer conjugate exhibited visually distinct color changes. A Qdots-based lateral flow immunoassay for detection of chloramphenicol in milk was developed and the Qdots-mAb/ CAP conjugate concentration of 7 nmol/L was the minimum amount that did not increase the LOD [97]. Another lateral flow strip biosensor based on MWCNTs for rapid and sensitive determination of aqueous mercury ions has also been described recently [3]. In this study, MWCNT-DNA was used as a recognition probe for the detection of Hg2+, and the accumulation of MWCNTDNA could be adopted for qualitative and quantitative analysis of the target Hg2+. Nevertheless, the amount of MWCNT-DNA conjugates optimized from 3 μL to 10 μL on the conjugation pad is a crucial factor directly affects the sensitivity. The results show 5 μL MWCNT-DNA conjugate exhibits maximum intensity for the control line, and hence, this volume was used for all the subsequent research.
5.3. The pH for the conjugate of the bio-recognition elements and signal transducersThe combination of the two elements has a great influence on the sensitivity of the LFA system and the pH can influence the combination. In a lateral flow immunoassay based on self-paired monoclonal antibody for rapid detection of Acidovorax avenae subsp. citrulli, the pH for the stable conjugation of AuNPs and McAb was optimized [127]. When the pH was 7.5, the color of the AuNPs solution was red and there was no discoloration or condensation. These results indicated that pH 7.5 is most suitable for conjugation of AuNPs and McAb. Another AuNPs-based LFA for the detection of Salmonella typhi in environmental water samples was developed recently. For stable conjugation of the AuNPs with the antibody, the pH of the AuNPs was optimized from 6 to10 [128], and pH 8 was optimized for the conjugation. There is a semi-quantitative lateral flow immunochromatographic strip for prostate acid phosphatase testing in which the pH value of AuNPs solution for labeling the antibody was adjusted to 8.2 with 0.1 mol/L K2CO3 [129].
5.4. The characteristics of the signal transducers (AuNPs)The signal transduction element is a very important factor in the test strip which would affect the response and then affect the detection sensitivity. The size and shape of AuNPs play an essential role in the lateral flow strip sensor based on AuNPs. Almost all AuNPs-based test strip sensor need to optimize the size of AuNPs. Previous studies have shown that the GNP size preventing aggregation and increasing detection sensitivity were between 20 nm and 40 nm [61, 129-131]. There is a signal enhancement lateral flow immunoassay based on dual AuNPs conjugates for detection of thrombin, which was achieved by using two GNP-DNA conjugates to increase the number of AuNPs accumulated on the test line [50]. After optimization, the signal response was found to be most pronounced when the 1st GNPs were 30 nm and the 2nd GNPs were 16 nm in diameter. In addition, a barcode semiquantitative lateral flow immunochromatographic strip for prostate acid phosphatase (PAP) was developed, which the antibody specific for PAP was labeled to AuNPs and another monoclonal antibody was immobilized on NC membrane to a barcode pattern, respectively [129]. The intensity of color signal was closely related to the size and quality of the AuNPs particles. For this study, the optimized size of AuNPs was 20 nm.
5.5. Type of the NC membranesOne of the most important elements of a lateral flow strip sensor is the nitrocellulose membrane. It was known that flow rates varied amongst different NC membrane types because of their different pore sizes and chromatographic performance, hence, the type of NC membranes could affect the reaction efficiency of the assay and result in difference of sensitivity [54]. To obtain high signal intensity, three kinds of commercial nitrocellulose membranes (Millipore HFB180, HFC180 and HFC135) were applied to the DNA-AuNPs network amplified LFA for detection of HBV [54]. As shown in the assay, lateral flow strips that use HFB18002 membrane performed best. Magnetic signals at T-lines on 4 NC membranes (Sartourius CN 140, Millipore HF 135, Whatman Prima 40 and Whatman F) were compared in a rapid detection of fish major allergen parvalbumin using superparamagnetic nanoparticle-based LFA [73]. Taking into account the test time and magnetic signal, CN 140 was selected as the most suitable NC membranes. There is a Qdots-based lateral flow immunoassay for detection of chloramphenicol in milk, they tested CNPC, CNPF, and CNPH NC membranes that have different pores sizes and flow rates [97]. The data show that the CNPC12 membrane was the most suitable to obtain the maximum fluorescence in the test line with the lowest standard deviation.
5.6. BuffersVarious buffers applied in the lateral flow strip biosensor influence on the sensitivity of the assay. During the fabrication of the biosensor and assay, several kinds of buffers were used: (1) Buffer that saturates the sample pad. (2) Buffer that disperses the detection elements conjugate. (3) Running buffer. Proper running buffer would minimize the nonspecific adsorption and increase the sensitivity and reproducibility of the sensor. The ingredient of the buffer was usually determined according to specific functions of buffer components. The percentage of sucrose is crucial in the detection system for it is a commonly used additive to keep DNA in its native state and to maintain the nucleic acid activity and aggregates with targets [58]. In a LFA for detection of thrombin based on aptamer and AuNPs, five varying percentages of sucrose were tested and 40 μL 2% sucrose was choose for further experiments [46]. In this assay, eight types of running buffers (1% PBST, PBS, 1% PBSB, 20 × SSC, 15 × SSC, 15 × SSC (1% BSA), 15 × SSC (5% BSA) and 10 × SSC) were used for optimization, and the largest peak area and the best S/N ratio were obtained when (15 × SSC (1% BSA)) was used. Another strip biosensor based on aptamer functionalized AuNPs for thrombin analysis optimized the running buffer. Several buffers, including Tris-HCl, PBS + 0.1% Tween, PBS, PBS + 1% BSA, 15 × SSC and 15 × SSC + 1% BSA were tested, and the results shown that the best performance was obtained with the 15 × SSC (1% BSA) buffer [45].
6. Conclusions and outlookThis review summarizes the main trends in the development of LFA test strips. In addition, we discuss the lateral flow test strip assay from various perspectives, focusing on the systems, biorecognition molecules, labels, detection targets, etc. Finally, we summarize the general optimization of the lateral flow test strip assay.
Aptamers show better versatility and effectiveness in the field of detection than antibodies, but the practical application of aptamer-based strip sensing and diagnostics is still lagging behind that of antibody-based strip tests. On one hand, flexibility of the aptamer structure may affect the stability of the aptamers, as the single-stranded nucleic acids can easily self-assembled to be structurally flexible under some specific conditions. Thus, in order to improve the sensitivity of the strip sensor, it is necessary to optimize the recognition conditions, to avoid or minimize occurrence of the nucleic acid self-folding when using the aptamer as a bio-recognition element in the lateral flow strip sensor. On the other hand, LFA itself has been verified some disadvantages including towing phenomena diffusion phenomena, and significant deviation owing to complicated and heterogeneous structure of nitrocellulose membrane, so different NC membrane will influence the sensitivity of the strip sensor. Moreover, most of the current test trips are qualitative or semi-quantitative due to technical difficulties in developing a fully quantified system. More attention should be paid on the application of strip readers, as they can improve the precision of the sensors by the system of CCD or PMT. At last, as there are many factors that cannot be controlled which leads to low reproducibility and instability, detailed optimization should be investigated when developing a LFA. At present and in the future, more attention is being paid on the research of multi-dimensional, multi-target, broad spectrum or class-specific detection systems [132] using lateral flow strip assay [60].
In short, the LFA strip sensors have a broad range of applications in clinical analysis and other fields, with the following advantages: time-saving, cost-effective, simpleness of operation, flexibility in configuration, and easiness to label. The present review can be envisaged to be helpful to the systematic understanding, rational design and wide application of the LFAs.
AcknowledgmentsThe authors are thankful for the financial support to the Key Program of Beijing Municipal Science and Technology Commission (No. D161100002116002), the National Key Research and Development Program of China (Nos. 2016YFF0203703, 2017YFB0306901), and the National Natural Science Foundation of China (No. 51673012).
| [1] |
B. Ngom, Y.C. Guo, X.L. Wang, D.B. Bi, Anal. Bioanal. Chem. 397 (2016) 1113-1135. |
| [2] |
J.H. Chen, S.G. Zhou, J.L. Wen, Anal. Chem. 86 (2014) 3108-3114. DOI:10.1021/ac404170j |
| [3] |
L. Yao, J. Teng, M.Y. Zhu, et al., Biosens. Bioelectron. 85 (2016) 331-336. DOI:10.1016/j.bios.2016.05.031 |
| [4] |
Z.Y. Fang, J. Huang, P.C. Lie, et al., Chem. Commun. 46 (2010) 9043-9045. DOI:10.1039/c0cc02782k |
| [5] |
D. Mazumdar, J.W. Liu, G. Lu, J.Z. Zhou, Y. Lu, Chemcommun. 46 (2010) 1416-1418. |
| [6] |
V.C. Özalp, D. Çam, F.J. Hernandez, et al., Analyst 141 (2016) 2595-2599. DOI:10.1039/C6AN00273K |
| [7] |
J.G. Bruno, Pathogens 3 (2014) 341-355. DOI:10.3390/pathogens3020341 |
| [8] |
C. Tuerk, L. Gold, Science 249 (1990) 505-510. DOI:10.1126/science.2200121 |
| [9] |
A.D. Ellington, J.W. Szostak, Nature 346 (1990) 818-822. DOI:10.1038/346818a0 |
| [10] |
G. Subash, Anal. Bioanal. Chem. 387 (2007) 171-182. |
| [11] |
S. Tombelli, M. Minunni, M. Mascini, Biosens. Bioelectron. 20 (2005) 2424-2434. DOI:10.1016/j.bios.2004.11.006 |
| [12] |
F. Ding, Y. Gao, X. He, Bioorg. Med. Chem. Lett. 27 (2017) 4256-4269. DOI:10.1016/j.bmcl.2017.03.032 |
| [13] |
S.M. Khoshfetrat, M.A. Mehrgardi, Bioelectrochemistry 114 (2017) 24-32. DOI:10.1016/j.bioelechem.2016.12.001 |
| [14] |
M. Heidelberger, Bacteriol. Rev. 3 (1939) 49-95. |
| [15] |
E.P. Meulenberg, Toxins 4 (2012) 244-266. DOI:10.3390/toxins4040244 |
| [16] |
M. Kuwahara, C.D. Maria, J. Nucl. Acid. 2012 (2012) 748913-748932. |
| [17] |
M. Kuwahara, C.D. Maria, Percept. Motor. Skill 1 (2012) 12-16. |
| [18] |
K.M. Song, M. Cho, H. Jo, et al., Anal. Biochem. 415 (2011) 175-181. DOI:10.1016/j.ab.2011.04.007 |
| [19] |
M. McKeague, A. Giamberardino, M.C. DeRosa, Advances in aptamer-based biosensors for food safety, in:V. Somerset (Ed.), Environmental Biosensors, Intech Inc., Rijeka, 2011, pp. 17-42.
|
| [20] |
P.M. Monatsh, Chemistry 140 (2009) 953-964. |
| [21] |
A.L. Chen, S.M. Yang, Biosens. Bioelectron. 71 (2015) 230-242. DOI:10.1016/j.bios.2015.04.041 |
| [22] |
Y.S. Kim, J.H. Kim, I.A. Kim, et al., Biosens. Bioelectron. 26 (2010) 1644-1649. DOI:10.1016/j.bios.2010.08.046 |
| [23] |
Y.N. Hou, J.F. Liu, M. Hong, et al., Biosens. Bioelectron. 92 (2017) 259-265. DOI:10.1016/j.bios.2017.02.024 |
| [24] |
J. Liu, D. Mazumdar, Y. Lu, Angew. Chem. 45 (2006) 7955-7959. |
| [25] |
M. Li, X.J. Zhou, W.Q. Ding, S.W. Guo, N.Q. Wu, Biosens. Bioelectron. 41 (2014) 889-893. |
| [26] |
C.H. Chung, J.H. Kim, J. Jung, B.H. Chung, Biosens. Bioelectron. 41 (2012) 827-832. |
| [27] |
R. Yamamoto, P.K.R. Kumar, Genes. Cells 5 (2000) 389-396. DOI:10.1046/j.1365-2443.2000.00331.x |
| [28] |
V.T. Nguyen, B.H. Lee, S.H. Kim, M.B. Gu, J. Biotechnol. 11 (2016) 843-849. DOI:10.1002/biot.201500433 |
| [29] |
D. Wu, T. Gao, L. Lei, et al., Anal. Chim. Acta 942 (2016) 68-73. DOI:10.1016/j.aca.2016.09.010 |
| [30] |
J.L. Li, K.X. Sun, Z.P. Chen, J.F. Shi, Biosens. Bioelectron. 89 (2017) 964-969. DOI:10.1016/j.bios.2016.09.078 |
| [31] |
Y.Y. Dong, Y. Xu, W. Yong, X.G. Chu, D.N. Wang, Crit. Rev. Food Sci. (2014) 1548-1561. |
| [32] |
D. Grate, C. Wilson, Proc. Natl. Acad. Sci. U. S. A. 96 (1999) 6131-6136. DOI:10.1073/pnas.96.11.6131 |
| [33] |
S.L. Stead, H. Ashwin, B. Johnston, et al., Anal. Chem. 82 (2010) 2652-2660. DOI:10.1021/ac902226v |
| [34] |
G. Lautner, Z. Balogh, V. Bardoczy, T. Meszarosb, R.E. Gyurcsanyi, Analyst 135 (2010) 918-926. DOI:10.1039/b922829b |
| [35] |
R. Bala, M. Kumar, K. Bansal, R.K. Sharma, N. Wangoo, Biosens. Bioelectron. 85 (2016) 445-449. DOI:10.1016/j.bios.2016.05.042 |
| [36] |
T.H. Nguyen, R. Pei, M. Stojanovic, Q. Lin, Sensor. Actuat. B-Chem. 155 (2011) 58-66. DOI:10.1016/j.snb.2010.11.024 |
| [37] |
T.H. Nguyen, R.J. Pei, M. Stojanovic, Q. Lin, Microfluid. Nanofluid. 6 (2009) 479-487. DOI:10.1007/s10404-008-0322-4 |
| [38] |
H.A. Oktem, G. Bayramoglu, V.C. Ozalp, M.Y. Arica, Biotechnol. Progr. 23 (2007) 146-154. DOI:10.1021/bp0602505 |
| [39] |
Ö. Kökpinar, J.G. Walter, Y. Shoham, F. Stahl, T. Scheper, Biotechnol. Bioeng. 108 (2011) 2371-2379. DOI:10.1002/bit.v108.10 |
| [40] |
S. Javaherian, M.U. Musheev, M. Kanoatov, M.V. Berezovsk, S.N. Krylov, Nucleic. Acids. Res. 37 (2009) e62. DOI:10.1093/nar/gkp176 |
| [41] |
A.C. Connor, L.B. McGown, J. Chromatogr. A 1111 (2006) 115-119. DOI:10.1016/j.chroma.2005.05.012 |
| [42] |
H.X. Liu, F.F. Zhan, F. Liu, et al., Biosens. Bioelectron. 62 (2014) 38-46. DOI:10.1016/j.bios.2014.06.020 |
| [43] |
B.B. Dzantiev, N.A. Byzova, A.E. Urusov, A.V. Zherdev, Trac-Trend. Anal. Chem. 55 (2014) 81-93. DOI:10.1016/j.trac.2013.11.007 |
| [44] |
M. Sajid, A.N. Kawde, M. Daud, J. Saudi. Chem. Soc. 19 (2015) 689-705. DOI:10.1016/j.jscs.2014.09.001 |
| [45] |
H. Xu, X. Mao, Q.X. Zeng, et al., Anal. Chem. 81 (2009) 669-675. DOI:10.1021/ac8020592 |
| [46] |
Q. Zhang, H.B. Qiu, F.Q. Tang, et al., Chem. Lett. 45 (2016) 289-290. DOI:10.1246/cl.151077 |
| [47] |
W.L. Zhou, W.J. Kong, X.W. Dou, et al., J. Chromatogr. B 1022 (2016) 102-108. DOI:10.1016/j.jchromb.2016.04.016 |
| [48] |
L.B. Wang, W.W. Ma, W. Chen, et al., Biosens. Bioelectron. 26 (2011) 3059-3062. DOI:10.1016/j.bios.2010.11.040 |
| [49] |
L. Anfossi, C. Giovannoli, G. Giraudi, et al., J. Agric. Food Chem. 60 (2012) 11491-11497. DOI:10.1021/jf3031666 |
| [50] |
G.Y. Shen, S.B. Zhang, X. Hu, Clin. Biochem. 46 (2013) 1734-1738. DOI:10.1016/j.clinbiochem.2013.08.010 |
| [51] |
R.H. Shyu, H.F. Shyu, H.W. Liu, S.S. Tang, Toxicon 40 (2002) 255-258. DOI:10.1016/S0041-0101(01)00193-3 |
| [52] |
W. Yang, X.B. Li, G.W. Liu, et al., Biosens. Bioelectron. 26 (2011) 3710-3713. DOI:10.1016/j.bios.2011.02.016 |
| [53] |
N.H.A. Raston, V.T. Nguyen, M.B. Gu, Biosens. Bioelectron. 93 (2017) 21-25. DOI:10.1016/j.bios.2016.11.061 |
| [54] |
Y. Gao, X.L. Deng, W. Wen, X.H. Zhang, S.F. Wang, Biosens. Bioelectron. 92 (2017) 529-535. DOI:10.1016/j.bios.2016.10.068 |
| [55] |
Y. Zhou, F.G. Pan, Y.S. Li, et al., Biosens. Bioelectron. 24 (2009) 2744-2747. DOI:10.1016/j.bios.2009.01.034 |
| [56] |
D.W. Li, S. Wei, Y. Hong, Y. Li, A.P. Deng, Biosens. Bioelectron. 24 (2009) 2277-2280. DOI:10.1016/j.bios.2008.11.004 |
| [57] |
X.X. Fu, R.S. Xie, J. Wang, et al., Toxins 9 (2017) 79-89. DOI:10.3390/toxins9030079 |
| [58] |
C.Y. Qin, W. Wen, X.H. Zhang, H.S. Gu, S.F. Wang, Analyst 140 (2015) 7710-7717. DOI:10.1039/C5AN01712B |
| [59] |
L. Naik, R. Sharma, B. Mann, et al., Food Chem. 219 (2017) 85-92. DOI:10.1016/j.foodchem.2016.09.090 |
| [60] |
Y.Q. Chen, Q. Chen, M.M. Han, et al., Biosens. Bioelectron. 79 (2016) 430-434. DOI:10.1016/j.bios.2015.12.062 |
| [61] |
N.F. Xu, L.G. Xu, W. Ma, et al., Food Agric. Immunol. 26 (2015) 635-644. DOI:10.1080/09540105.2014.998640 |
| [62] |
W.B. Shim, M.J. Kim, H. Mun, M.G. Kim, Biosens. Bioelectron. 62 (2014) 288-294. DOI:10.1016/j.bios.2014.06.059 |
| [63] |
L.M. Hu, K. Luo, J. Xia, et al., Biosens. Bioelectron. 91 (2017) 95-103. DOI:10.1016/j.bios.2016.12.030 |
| [64] |
C.H. Wu, L.M. Hu, J. Xia, et al., J. Dairy. Sci. 100 (2017) 2501-2511. DOI:10.3168/jds.2016-12065 |
| [65] |
B. Wang, Y.F. Chen, Y.Y. Wu, et al., Biosens. Bioelectron. 78 (2016) 23-30. DOI:10.1016/j.bios.2015.11.015 |
| [66] |
X.P. Li, D.L. Lu, Z.H. Sheng, et al., Talanta 100 (2012) 1-6. DOI:10.1016/j.talanta.2012.08.041 |
| [67] |
M. Oroval, E. Climent, C. Coll, et al., Chem. Commun. 49 (2013) 5480-5482. DOI:10.1039/c3cc42157k |
| [68] |
M. Colombo, S.C. Romero, M.F. Casula, et al., Chem. Soc. Rev. 41 (2012) 4306-4334. DOI:10.1039/c2cs15337h |
| [69] |
J.H. Gao, H.W. Gu, B. Xu, Accounts. Chem. Res. 42 (2009) 1097-1107. DOI:10.1021/ar9000026 |
| [70] |
C. Sun, J.S.H. Lee, M.Q. Zhang, Adv. Drug. Deliver. Rev. 60 (2008) 1252-1265. DOI:10.1016/j.addr.2008.03.018 |
| [71] |
M. De, P.S. Ghosh, V.M. Rotello, Adv. Mater. 20 (2008) 4225-4241. DOI:10.1002/adma.v20:22 |
| [72] |
N.M. Nor, K.A. Razak, S.C. Tan, R. Noordin, J. Alloy. Compd. 538 (2012) 100-106. DOI:10.1016/j.jallcom.2012.05.053 |
| [73] |
C. Zheng, X.C. Wang, Y. Lu, Y. Liu, Food Control. 26 (2012) 446-452. DOI:10.1016/j.foodcont.2012.01.040 |
| [74] |
S. Handali, M. Klarman, A.N. Gaspard, et al., Clin. Vaccine. Immunol. 17 (2010) 631-637. DOI:10.1128/CVI.00511-09 |
| [75] |
K. Taton, D. Johnson, P. Guire, E. Lange, M. Tondra, J. Magn. Magn. Mater. 321 (2009) 1679-1682. DOI:10.1016/j.jmmm.2009.02.113 |
| [76] |
Y.Y. Wang, H. Xu, M. Wei, et al., Mat. Sci. Eng. C-Mater. 29 (2009) 714-718. DOI:10.1016/j.msec.2009.01.011 |
| [77] |
F.M. Liu, H.L. Zhang, Z.H. Wu, et al., Talanta 161 (2016) 205-210. DOI:10.1016/j.talanta.2016.08.048 |
| [78] |
R. Greenwald, J. Esfandiari, S. Lesellier, et al., Diagn. Micr. Infec. Dis. 46 (2003) 197-203. DOI:10.1016/S0732-8893(03)00046-4 |
| [79] |
X. Mao, W. Wang, T.E. Du, Talanta 114 (2013) 248-253. DOI:10.1016/j.talanta.2013.04.044 |
| [80] |
S. Lee, S. Mehta, D. Erickson, Anal. Chem. 88 (2106) (2017) 8359-8363. 10.1021/acs.analchem.6b01828
|
| [81] |
E.B. Bahadır, M.K. Sezginturk, Trac-Trend. Anal. Chem. 82 (2016) 286-306. DOI:10.1016/j.trac.2016.06.006 |
| [82] |
M. Beggs, M. Novotny, S. Sampedro, Clin. Chem. 36 (1990) 1084-1085. |
| [83] |
J.M. Park, H.W. Jung, Y.W. Chang, et al., Anal. Chim. Acta 853 (2015) 360-367. DOI:10.1016/j.aca.2014.10.011 |
| [84] |
L. Anfossi, C. Baggiani, C. Giovannoli, G. D'Arco, G. Giraudi, Anal. Bioanal. Chem. 405 (2013) 467-480. DOI:10.1007/s00216-012-6033-4 |
| [85] |
R. Krska, A. Molinelli, Anal. Bioanal. Chem. 39 (2009) 67-71. |
| [86] |
I. Bazin, E. Nabais, M.L. Ferber, Toxins 2 (2010) 2230-2241. DOI:10.3390/toxins2092230 |
| [87] |
R. Köppen, M. Koch, D. Siegel, et al., Appl. Microbiol. Biotechnol. 86 (2010) 1595-1612. DOI:10.1007/s00253-010-2535-1 |
| [88] |
I.Y. Goryacheva, S.D. Saeger, S.A. Eremin, C.V. Peteghem, Food Addit. Contam. 24 (2007) 1169-1183. DOI:10.1080/02652030701557179 |
| [89] |
Y.J. Cho, D.H. Lee, D.O. Kim, et al., J. Agri. Food Chem. 53 (2005) 8447-8451. DOI:10.1021/jf051681q |
| [90] |
S. Won-Bo, B.B. Dzantiev, S.A. Eremin, D.H. Chung, J. Microbiol. Biotechnol. 19 (2009) 83-89. |
| [91] |
W.H. Lai, D.Y.C. Fung, Y. Xu, R.R. Liu, Y.H. Xiong, Food Control. 20 (2009) 791-795. DOI:10.1016/j.foodcont.2008.10.007 |
| [92] |
X.H. Wang, T. Liu, N. Xu, Y. Zhang, S. Wang, Anal. Bioanal. Chem. 389 (2007) 903-911. DOI:10.1007/s00216-007-1506-6 |
| [93] |
EFSA, EFSA J. 7 (2009) 1-23. |
| [94] |
W. Jawaid, K. Campbell, K. Melville, et al., Anal. Chem. 87 (2015) 5324-5332. DOI:10.1021/acs.analchem.5b00608 |
| [95] |
A. Komano, H. Maruko, H. Sekiguchi, Y. Seto, Forensic. Toxicol. 29 (2011) 38-43. DOI:10.1007/s11419-010-0102-1 |
| [96] |
X.H. Xia, Y. Xu, R.Q. Ke, et al., Anal. Bioanal. Chem. 405 (2013) 7541-7544. DOI:10.1007/s00216-013-7210-9 |
| [97] |
A.N. Berlina, N.A. Taranova, A.V. Zherdev, Y.Y. Vengerov, B.B. Dzantiev, Anal. Bioanal. Chem. 405 (2013) 4997-5000. DOI:10.1007/s00216-013-6876-3 |
| [98] |
K. Li, L. Liu, C. Xu, X. Chu, Anal. Sci. 23 (2007) 1281-1284. DOI:10.2116/analsci.23.1281 |
| [99] |
X. Yu, Y. Lu, Inorg. Chem. 53 (2014) 1925-1942. DOI:10.1021/ic4019103 |
| [100] |
J.H. Chen, X.M. Zhou, L.W. Zeng, Chem. Commun. 49 (2013) 984-986. DOI:10.1039/C2CC37598B |
| [101] |
A.M. López, J. Marzo, D.A. Pons, A. Blake Merkoçi, Biosens. Bioelectron. 47 (2013) 190-198. DOI:10.1016/j.bios.2013.02.031 |
| [102] |
K. Abe, K. Nakamura, T. Arao, et al., J. Sci. Food Agric. 91 (2011) 1392-1397. DOI:10.1002/jsfa.v91.8 |
| [103] |
X. Liu, J.J. Xiang, Y. Tang, et al., Anal. Chim. Acta 745 (2012) 99-105. DOI:10.1016/j.aca.2012.06.029 |
| [104] |
P.S. Ullrich, J. Rudolf, P. Ansari, et al., Anal. Bioanal. Chem. 395 (2009) 69-81. DOI:10.1007/s00216-009-2715-y |
| [105] |
H.W. Wen, W.B. Wysocki, T.R. DeCory, R.A. Durst, Anal. Bioanal. Chem. 382 (2005) 1217-1226. DOI:10.1007/s00216-005-3292-3 |
| [106] |
H.W. Wen, W.B. Wysocki, T.R. DeCory, A.J. Baeumner, R.A. Durst, Eur. Food Res. Technol. 221 (2005) 564-569. DOI:10.1007/s00217-005-1202-8 |
| [107] |
C.R. Suri, R. Boro, Y. Nangia, et al., Trac-Trend. Anal. Chem. 28 (2009) 29-39. DOI:10.1016/j.trac.2008.09.017 |
| [108] |
Y.A. Cho, Y.J. Kim, B.D. Hammock, Y.T. Lee, H.S. Lee, J. Agric. Food Chem. 51 (2003) 7854-7860. DOI:10.1021/jf0346915 |
| [109] |
J. Kaur, K.V. Singh, R. Boro, et al., Environ. Sci. Technol. 41 (2007) 5028-5036. DOI:10.1021/es070194j |
| [110] |
Y. Liu, A.H. Wu, J. Hu, et al., Anal. Biochem. 483 (2015) 7-11. DOI:10.1016/j.ab.2015.04.022 |
| [111] |
W.C. Andersen, S.B. Turnipseed, C.M. Karbiwnyk, et al., J. Agric. Food Chem. 56 (2008) 4340-4347. DOI:10.1021/jf800295z |
| [112] |
T. Le, P.F. Yan, J. Xu, Y.J. Hao, Food Chem. 138 (2013) 1610-1615. DOI:10.1016/j.foodchem.2012.11.077 |
| [113] |
Z.Z. Wang, D.J. Zhi, H.L. Zhang, et al., Int. J. Nanomed. 9 (2014) 1699-1707. |
| [114] |
Y.H. Zhong, Y.J. Chen, L. Yao, et al., Microchim. Acta 183 (2016) 1989-1994. DOI:10.1007/s00604-016-1812-9 |
| [115] |
F.X. Sun, L.Q. Liu, W.W. Ma, et al., Inter. J. Food Sci. Tech. 47 (2012) 1505-1510. DOI:10.1111/j.1365-2621.2012.02998.x |
| [116] |
G.A. Mitchell, G. Dunnavan, Anim. Sci. 76 (1998) 208-211. DOI:10.2527/1998.761208x |
| [117] |
J. Pleadin, A. Vulić, N. Perši, N. Vahčić, Meat Sci. 86 (2010) 733-737. DOI:10.1016/j.meatsci.2010.06.013 |
| [118] |
F. Ramos, A. Cristino, P. Carrola, et al., Anal. Chim. Acta 483 (2003) 207-213. DOI:10.1016/S0003-2670(02)01020-6 |
| [119] |
J.F. Martínez-Navarro, Lancet 336 (1990) 1311-1311. |
| [120] |
X.L. Qu, H. Lin, S.Y. Du, et al., Food Anal. Method 9 (2016) 2531-2540. DOI:10.1007/s12161-016-0442-5 |
| [121] |
J.Y. Wang, L. Zhang, Y.Y. Huang, et al., Sci. Rep. 7 (2017) 41419-41427. DOI:10.1038/srep41419 |
| [122] |
P.L. Wang, R.G. Wang, W. Zhang, X.O. Su, H.F. Luo, Biosens. Bioelectron. 77 (2016) 866-870. DOI:10.1016/j.bios.2015.10.053 |
| [123] |
G.W. Khaemba, B.N. Tochi, D. Mukunzi, et al., Food Agric. Immunol. 27 (2016) 111-127. DOI:10.1080/09540105.2015.1079598 |
| [124] |
F.E. Ahmed, Trends. Biotechnol. 20 (2002) 215-223. DOI:10.1016/S0167-7799(01)01920-5 |
| [125] |
H. Akiyama, T. Watanabe, H. Kikuchi, et al., J. Food Hygiene Soc. Jap. 47 (2006) 111-114. DOI:10.3358/shokueishi.47.111 |
| [126] |
A.N. Berlina, A.V. Zherdev, C.L. Xu, S.A. Eremin, B.B. Dzantiev, Food Control. 73 (2016) 247-253. |
| [127] |
H.J. Zeng, W.B. Guo, B.B. Liang, et al., Anal. Bioanal. Chem. 408 (2016) 6071-6078. DOI:10.1007/s00216-016-9715-5 |
| [128] |
J. Singh, S. Sharma, S. Nara, Anal. Methods 7 (2015) 9281-9288. DOI:10.1039/C5AY02271A |
| [129] |
C. Fang, Z.C. Chen, L. Li, J.H. Xia, Analysis 56 (2011) 1035-1040. |
| [130] |
S. Lou, J.Y. Ye, K.Q. Li, A.G. Wu, Analyst 137 (2012) 1174-1181. DOI:10.1039/C2AN15844B |
| [131] |
Z. Tian, L.Q. Liu, C.F. Peng, Z.X. Chen, C.L. Xu, Food Agric. Immunol. 20 (2009) 1-10. DOI:10.1080/09540100802621017 |
| [132] |
L.L. Tan, Z.B. Chen, Y. Zhao, et al., Biosens. Bioelectron. 85 (2016) 414-421. DOI:10.1016/j.bios.2016.05.038 |
 2018, Vol. 29
2018, Vol. 29