Optical sensors are widely used in the field of analytical sensing and optical imaging due to their high sensitivity, fast response time, and technical simplicity [1-3]. In recent years great strides has been taken in fluorescence sensor research. Development of this area has produced important fluorescence sensing systems, which provide a reliable fluorescent response for the analysis of environmental or biological targeted analytes. In some cases small molecule based sensors are employed, allowing the highly selective quantitative determination of a given analyte. The sensing targets range from cations to anions as well as small biologically active molecules, to tumor micro-environment-related parameters, which include viscosity, polarity, temperature, hypoxia, etc. [4, 5].
Due to the vital roles cations and anions play in human life and in nature, their detection is of great interest and importance to chemists, biologists and environmentalists [6]. Therefore, the development of methodologies for anion and cation recognition and sensing within biological and environmental important species, has emerged as a signi ficant target in the field of chemical sensors [7, 8]. In last 15 years, a large number of optical sensors have been developed for anion and cation detection [9], in which most reported sensors follow three main approaches: hydrogen bond interaction, displacement approach and reaction-based chemodosimeter paradigm [10].
The development of biologically active molecular-recognition and sensing systems for biologically important targets, including amino acid, nucleotides, thiol-containing small molecules such as cysteine (Cys), homo-cysteine (Hcy), and glutathione (GSH) has also received considerable attention in recent ten years [11-13].
Cancer is an extremely aggressive disease, with a high incidence and death rate. Early stage detection of preinvasive and metastatic tumor cells in patients is vital for tumor therapy and improvement of survival rates. This implies that fluorescence sensors can be utilized as a non-invasive, real-time, and high-resolution modality for the early diagnosis of tumors [14]. Only recently, new and exciting efforts have been made in the design, application, and development of small molecular-based fluorescent imaging agents and fluorescent theranostics for tumor diagnosis and therapy. The reports detail strategies for creating multifunctional compounds possessing speci fic targeting ligands and fluorophores. These units were linked using a spacer, which promotes cleavage after endocytosis under a selective chemical reaction, releasing the masked cytotoxic drug into tumor cells [15].
This review aims to compile certain examples published in last ten years, mainly by our group and some related research groups, which are connected with anion and cation sensing, biological neutral molecular-recognition and tumor micro-environment- related parameters sensing. Due the vast number of published works in this field, only some typical examples are commented. In retrospect, the development of fluorescence sensors underwent three main eras, all of which will be discussed in this review, viz., ions sensing, biological neutral molecule sensing and cancer research-related medical integration. Particular emphasis is placed on the chemical features of these sensors, their design strategies, optical properties and biological applications.
2. Cationic sensor 2.1. Cadmium ion sensorQian and coworkers synthesized a series of derivatives from 1, 8- naphthyridine [16], including, compound 1 (Fig. 1), the first naphthyridine photoinduced electron-transfer (PET) sensor, possessing a speci fic Cd2+ signal with fluorescent enhancement and red-shift. The spectra of compound 1 shows that it can form binuclear complexes with Cd2+, in which the process of capturing Cd2+ ions was divided into two steps. Firstly, two nitrogen atoms of naphthyridine capture one Cd2+ ion, resulting in an electron density decrease of the fluorophore. Then the accumulated piperazines offered an appropriate cage for another Cd2+ ion, which could reduce complexation through the PET process. Therefore, when the concentration of Cd2+ was low, the fluorescent was gradually red-shifted with a decrease in fluorescence. As the concentration progressed higher, a new band at 458 nm appeared with a red-shift. A binuclear complex structure was con firmed using Hyperchem software.

|
Download:
|
| Fig. 1. Chemical structures of compound 1 and proposed sensing mechanism to Cd2+. | |
2.2. Copper ion sensors
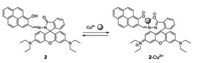
In 2009, Yoon's group synthesized a new rhodamine-based derivative, in which the pyrene moiety operates as a ratiometric and "off-on" chemosensor for Cu2+ [17]. As expected, the pyrene moiety was successfully used as a source of ratiometric changes in compound 2 (Fig. 2). By examining a single crystal of compound 2, its unique spirolactam could be observed. In comparison with other pyrene-based derivatives, it was determined that with addition of Cu2+ a weak fluorescence quench of pyrene and a large fluorescence enhancement as well as a colorimetric change of spirolactam ring-opening occurred. Therefore, compound 2 may be utilized as a chemosenser as it exhibited a slight fluorescence increase with a colorimetric change towards Cu2+ ions.
|
Download:
|
| Fig. 2. Structure of compound 2 and its response mechanism to Cu2+. | |
In order to enhance the ratiometric fluorescence signal output towards Cu2+, in 2010, Kim's group expanded this research by synthesizing a new rhodamine-based derivative (compound 3), bearing a 1, 8-naphthalimide group (Fig. 3) [18]. This moiety replaced the pyrene moiety as a source of ratiometric changes. Compound 3 worked as a dual-mode Cu2+-selective sensor via the rhodamine ring-opening approach and ratiometric displacement. The addition of Zn2+ generated 3-Zn2+ complexes, however the introduction of Cu2+ results in the expulsion of Zn2+ displaying a colorimetric and "off-on" fluorescent signal output for Cu2+. These results support that compound 3 was a dual-mode Cu2+ selective chemosensor with two mechanisms.

|
Download:
|
| Fig. 3. Chemical structure of compund 3 and proposed off/on sensing mechanism. | |
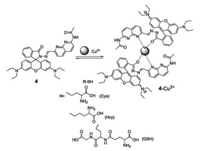
In 2012, Zhang and coworkers designed and synthesized a 1, 8- naphthyridine modi fied rhodamine B derivative (compound 4, Fig. 4), which can selectively detect Cu2+ with a dramatic color change from colorless to pink [19]. Due to the relatively stronger binding ability of Cys/Hcy/GSH and Cu2+, over that of compound 4 sensitivity toward Cu2+, resulting in a 4-Cu2+ complex, contributed to high sensitivity and selectivity in the detection of Cys/Hcy/GSH. Hence, the 4-Cu2+ complex was a selective chemosensor for the detection of Cys, Hcy, and GSH, over other naturally occurring α- amino acids in a 70% aqueous solution, displaying a color change from pink to colorless. These color changes throughout the process, from colorless to pink and then back to colorless, are all easily observed by the naked eye.
|
Download:
|
| Fig. 4. Structure of compound 4 and the plausible coordination mechanism of 4 and Cu2+. | |
2.3. Zinc ion sensors
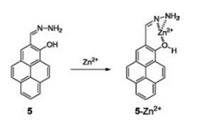
In 2010, Yoon's group designed and synthesized a simple and effective fluorescent sensor (compound 5, Fig. 5), based on hydrazine-pyrene, for Zn2+ detection [20]. A common occurrence in many Zn2+ sensors is the simultaneous responsiveness of Ca2+, which greatly interferes in the accuracy of Zn2+ sensing. In the case of compound 5, the two nitrogens on the5hydrazone moiety as well as phenolic oxygen participated in Zn2+ binding, excluding the influence of Ca2+. The accumulated data demonstrated that compound 5 was a highly selective Zn2+ sensor. Furthermore, the group applied these sensing properties of compound 5 for intracellular Zn2+ fluorescent imaging in HaCaT cells.
|
Download:
|
| Fig. 5. Structure of compound 5 and its response mechanism to Zn2+. | |
We examined a synthesized 1, 8-naphthalimide derivative (compound 6, Fig. 6) as a new turn-6on fluorescent sensor for the detection of Zn2+ ion at pH 7.4 in aqueous media [21]. The reaction mechanism involved the replacement of O-H group protons with a zinc ion at the binding site, resulting in fluorescence production being blocked in the PET process. A 13-fold increase in fluorescence intensity with a 38 nm red-shift was achieved in the detection of Zn2+. In addition, sensor 6 was successfully utilized for cell imaging of Zn2+ ion. It was discovered that in A549, BEAS-2B, CHO, Hela, and HepG2 cells, the fluorescence emission color produced in the cell nucleus was different from that produced in the cytoplasm. Fluorescence imaging in cytokinesis-block micronucleus (CBMN) assay was also carried out for CHO cells using compound 6 and Zn2+ ion as the imaging agents. It was deduced that 6-Zn2+ agent was a nucleic6acid selective stain, which could be applied in micronucleus assays in different kinds of cell lines.

|
Download:
|
| Fig. 6. Structure of compound 6 and the proposed binding mechanism of compound 6 with Zn2+. | |
2.4. Mercury ion sensors
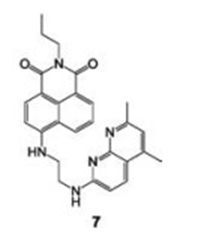
In the same year, Zhang et al., syanthesized compound 7, a bottom-modi fied (4-position) naphthalimide derivative (Fig. 7), in which 1, 8-naphthyridine was the binding site [22]. Compound 7 was the first 1, 8-naphthyridine-modi fied naphthalimide-based sensor that can selectively detect Hg2+ ions in organic aqueous solutions. Upon addition of Hg2+, the fluorescence of compound 7 slowly increased with a 13 nm blue-shift. A fluorescence color change from yellow to green-yellow 7 observed. The Job's plot and mass con firmed the 1:1 stoichiometry formation of 7-Hg2+ complex, which also strongly supports the above conclusions.
|
Download:
|
| Fig. 7. Chemical structure of compound 7. | |
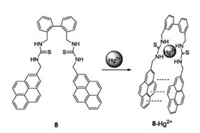
In order to further evaluate more biological applications of Hg2+-selective fluorescent chemosensor, we prepared compound 8 (Fig. 8), in which pyren8e was the fluorophore [23]. A sandwich-stacking binding mode was formed during the binding process, which gave a 22-fold increase in the excimer fluorescence at 490 nm. In the presence of Hg2+, the two thiourea units of sensor 8 are fully separated. Binding occurs through the -NH of the thiourea groups, instead of the thione groups. Sensor 8 was successfully applied in fluorescence imaging of BEAS-2B and CHO cells and C. elegans. More importantly, it could be used as a fluorescence agent to trace the enrichment and distribution of Hg2+ ions during zebra fish development in a simulated polluted environment.
|
Download:
|
| Fig. 8. Structure of compound 8 and the plausible coordination mechanism of 8 and Hg2+. | |
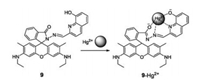
As the demand of convenient and rapid detection of Hg2+ is growing, a naked-eye detection is very necessary for the Hg2+ sensors. Therefore, we synthesized a simple and easy to prepare rhodamine-based sensor (compound 9, Fig. 9) for Hg2+-selective colorimetric and fluorescent sensing [24]. Upon addition of an increasing amount of Hg2+, the sensor 9 selectively detects Hg2+ with a color change from colorless to pink, which can be easily observed. In addition, a notable fluorescence enhancement of up to 300-fold with a bright yellow-green emission appeared. Compound 9 was successfully applied9in the in vivo imaging of Hg2+ in Spill 2 cells and C. elegans. This approach provides a sensitive and accurate method for the estimation of Hg2+ in environmental, tobacco and biological applications.
|
Download:
|
| Fig. 9. Structure of compound 9 and the proposed binding mechanism of compound 9 with Hg2+. | |
2.5. Iron ion sensors
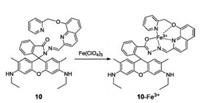
In 2015, we synthesized a rhodamine-based and "turn-on" pattern Fe3+-selective fluorescent sensor (compound 10, Fig. 10) [25]. It is well known that many Fe3+-based probes also respond to Fe2+, therefore, high selectivity towards Fe3+ over Fe2+ is crucial. In compound 10, pyridine and quinoline10are typically chosen as the ligating atom's donators. The binding of sensor 10 with Fe3+ can trigger an increase in fluorescence emission, thereby forming a "turn-on" fluorescent sensing system. Furthermore, sensor 10 exhibited excellent selectivity for Fe3+ with a 62-fold enhancement in fluorescence intensity, excluding any Fe2+ interference. The fluorescent discrimination of Fe3+ and Fe2+ guaranteed the accurate sensing of Fe3+ in BEAS-2B, HeLa cells and C. elegansMoreover, it was also used to elucidate Fe3+ enrichment and exchange infected hemichannel-closed Sf9 cells by innexin3 (Inx3).
|
Download:
|
| Fig. 10. Structure of compound 10 and its response mechanism to Fe3+. | |
2.6. Chromium ion sensor
Due to the remarkable optical properties and chemical stability of the rhodamine group, in 2017, our group investigated other rhodamine-based sensors, such as compound 11 (Fig. 11), for the detection of chromium ions [26]. Sensor 11 can selectively detect Cr3+ ions by colorimetric and fluorescent responses. Upon addition of 150 equiv. of Cr3+ ions, the emission intensity of sensor 11 at 556 nm increased 13-fold giving a color change from colorless to pink. Furthermore, sensor 11 was successfully utilize1d1 in vivo for Cr3+ fluorescence imaging of C. elegans.

|
Download:
|
| Fig. 11. Structure of compound 11 and the plausible coordination mechanism of 11 and Cr3+. | |
2.7. Aluminium ion sensors
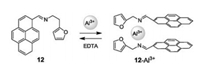
In order to examine the detection of Al3+, a low toxic and non heavy metal, Yao et al., ynthesized chemosensor 12, in a one-step reaction, which possessed pyrene and furan moieties (Fig. 12) [27]. Upon addition of a small amount of Al3+, the imine groups and O atoms from two sensors formed a complex with one Al3+ ion, resulting in two pyrene moieties folded face to face. This kind of conformational changes led to a fluorescence change from a weak blue to a strong green color. Whereas the color of the solution turned from colorless to faint yellow.
|
Download:
|
| Fig. 12. Chemical structure of compound 12 and proposed sensing mechanism to Al3+. | |
2.8. Gold ion sensor
Due to gold's low reactivity and its natural existence as an elementary substance, there is a few fluorescent sensor for Au3+ sensing. Feng's group designed and synthesized a new colorimetric and fluorescent sensor (compound 13, Fig. 13) for such a task [28]. Utilizing coumarin, as a fluorophore, and terminal ethynyl, as a recognition moiety, they were connected via an amide bond. Sensor 13 coordinated with Au3+, triggered a cascade reaction resulting in oxazolecarbaldehyde formation, and a strong blue fluorescence being observed. The color of the solution changed from yellow to colorless, qualify compound 13 as a Au3+ sensor.

|
Download:
|
| Fig. 13. Chemical structure of compound 13 and Au3+-induced reaction mechanism to 13. | |
2.9. Palladium ion sensors
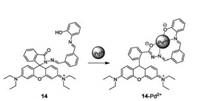
Palladium is widely used in electronics, dentistry, medicine, hydrogen puri fication, chemical applications and groundwater treatment. Hence, the requirement of rapid Pd2+ detection is becoming more and more necessary. Therefore, in 2016 Yang and coworkers designed a new selective and sensitive "off-on" sensor (compound 14) (Fig. 14) based on rhodamine for detecting Pd2+ [29]. With the addition of Pd2+, the hydroxyl O, imino N, benzoyl hydrazine N and O atoms bind to Pd2+, causing ring opening of the rhodamine spirolactam by chelation. The orange fluorescence increased quickly and the color of solution changed from colorless to pink. In addition, compound 14 was successful applied to fluorescence imaging in HepG2 living cells for Pd2+ sensing, which exhibited its value in biological systems.
|
Download:
|
| Fig. 14. Structure of compound 14 and its bonding sites for Pd2+. | |
3. Anion sensors 3.1. Tetrameric vanadate sensor
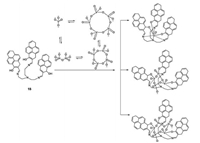
In 2011, Yoon's group synthesized tris(2-((ethylimino)methyl) pyren-1-ol)amine (compound 15, Fig. 15) as the first tetrameric vanadate fluorescence sensor [30]. In aqueous solution, the vanadate hydrolyzed giving both mono- and poly-nuclear species, such as mono-, di-, tetra-, and penta-nuclear compounds. In the sensing process, first compound 15 rapidly binds to V1, V4 and V5 simultaneously. The color of solution changed from pink to yellow, emitting a strong yellow-green fluorescence with a 30-fold enhancement. After the equilibrium of the oligomeric vanadates a shift towards V4 was observed, once again changing the color of solution from yellow to orange, with a 50-fold enhancement of yellow fluorescence emission. Therefore, the entire binding process was successfully accomplished in two steps: distinct colorimetric changes and "off-on" fluorescent enhancement.
|
Download:
|
| Fig. 15. Structure of compound 15 and its binding mechanism with different vanadate species. | |
3.2. Fluorinion sensors
In 2013, we prepared and spectroscopically characterized a highly selective and sensitive colorimetric signal-responding anion-sensor, sensor 16 (Fig. 16), based on 1, 8-naphthalimide [31]. Sensor 16 can selectively respond to fluoride (yellow-to-red color change), acetate (yellow-to-orange color change) and hydroxyl (yellow-to-brown color change) anions in DMSO. All these distinct changes are due to the formation of different hydrogen bonds between sensor 16 and the anions (F-, AcO-, OH-) whilst deprotonating the hydroxyl moiety in the binding group, which was associated with the enhancement in the push-pull effect of the internal charge-transfer transition. Two different processes were observed in the titration of F-, as shown in Fig. 16. This visual detection system provided a simple and rapid method for the detection of fluoride, acetate and hydroxyl anions without interferences from other halide anions or common anions.

|
Download:
|
| Fig. 16. Structure of 16 and its proposed sensing mechanisms to different anions. | |
Fluoride ion is a type of inorganic anion, which in low concentrations is safe for dental healthcare, but sustained ingestion of a certain amount is dangerous. In 2016, Liu developed a novel chromogenic and fluorescent chemosensor (compound 17) (Fig. 17) for the select1iv7e detection of F- [32]. A coumarin containing a CF3 group was used as the fluorophore, linked to phthalic acid with one protected carboxylic acid. Upon contact with F-, the ester bonds of compound 17 self-immolative linker cleavage, releasing the fluorophore. As a result, a strong green fluorescence was detected and the color of the solution changed from colorless to yellow.

|
Download:
|
| Fig. 17. Chemical structure of compound 17 and its mechanism involving the fluoride deprotonation. | |
3.3. Pyrophosphate sensor
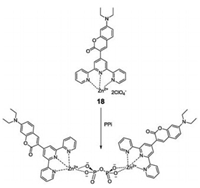
In 2016, a coumarin-based terpyridine-zinc complex (compound 18, Fig. 18) was designed18for sensing pyrophosphate in aqueous media (CH3CN-HEPES) [33]. The terpyridine moiety was introduced into sensor 18 as a binding site to first form a stable complex with Zn2+. Where, upon the addition of PPi, multiple- hydrogen bonds formed between the Zn2+-DPA binding center and the hydroxyl groups of PPi with a 2:1 formation mode. This resulted in an enhancement of fluorescence intensity at 505 nm, with the reaction mixture changing from colorless to pink. In addition, sensor 18was successful used to fl1u8orescence imaging of PPi in Hi5 insect cells and C. elegans.
|
Download:
|
| Fig. 18. Structure ofcompound 18 and the proposedbinding mechanism of 18 with PPi. | |
3.4. Phosphate ion sensors
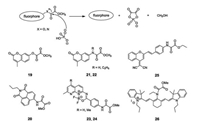
The phosphate ion (Pi), an important down-stream metabolic product of ATP, ADP, GTP etc., has been reported to widely participate in signaling and regulating biological processes. However, unlike those derived from ATP and PPi, sensors for selective recognition of inorganic phosphate, especially the phosphate anion, have been explored to a much lesser extent, due to the selectivity for sensing Pi is always hindered by the presence of structurally similar anions in cells. Owing to the significant advantages held by chemodosimeters, we designed a strategy utilizing a predesigned Pi induced reaction, which is not promoted by ATP, AMP, or PPi, to serve as the basis for sensing this important anionic species (Fig. 19).
|
Download:
|
| Fig. 19. Structures of compounds 19 — 26 and the proposed sensing mechanism of 19 — 26 with Pi. | |
Chemodosimeter 19 (Fig. 19), the first inorganic Pi targeted colorimetric and fluorescent probe based on the sensing mechanism shown in Fig. 19, contains an oxalate moiety linked via an ester bond to the hydroxyl group of coumarin fluorophore [34]. After adding Pi, the ester bond of compound 19 broke and released coumarin fluorophore, leading to a colorimetric change associated with a 62 nm red-shift in the UV–vis absorptionmaximum and upto a 780-fold enhancement in the fluorescence intensity. As sensor 19 can monitor Pi production in vivo caused by apyrase-catalyzed ATP hydrolysis, it was therefore successfully applied to fluorescence in vivo imaging of exogenous and endogenous Pi in Hela cells and C. elegans. Sensor 19 was the first fluorescence Pi sensor utilized to show that the apoptosis of hemichannel-closed Sf9 cells was caused by Inx3 promoted dephosphorylation of Akt (RAC serine/threonineprotein kinase), leading to an elevation of the concentration of Pi.
In order to enhance the selectivity and deepen the exploration of the fluorescent properties of Pi probes, we later designed a series of sensors based on different fluorophores with above sensing mechanism, such as 1, 8-naphthalimide based sensor 20, coumarin based sensors 21 and 22, 1, 8-naphthyridine-based boron complexes sensors 23 and 24, derivative of dicyanomethylene-4H-chromene sensor 25, cyanine based sensor 26 (Fig. 19). In this collection of probes, the amido was used to replace the hydroxyl group in order to be more sensitive to Pi. Upon addition of Pi, the amido units were cleaved releasing the corresponding fluorophores, which led to changes in the absorption and fluorescence spectra. In the presence of Pi, fluorescence emission of compound 20 underwent a 79 nm red-shift along with a highly significant ratiometric. These spectral changes were accompanied by a dramatic color change, from colorless to light yellow, which could be perceived by the naked eye. Sensor 20 was successfully applied to stain CHO cells and C. elegans for the detection of Pi generation. In hemichannel-closed Sf9 cells, sensor 20 traced the process of generation and accumulation of Pi along with ratiometric fluorescence changes [35].
Compound 21 displayed a remarkable fluorescence enhancement, up to 23-fold at 438 nm in DMSO-HEPES buffer (6:4, v/v, 0.02 mol/L, pH 7.4), with an accompanying turn-on blue fluorescence visually detected. In DMSO, compound 22 showed a 20-fold increase in the fluorescence intensity with the addition of Pi. These results suggested that Pi-triggered acetamide bond cleavage resulted in the release of the blue fluorophore coumarin [36].
Dramatic color changes from colorless to light yellow were observed for Pi solutions with the addition of sensor 23 and 24 in DMSO-HEPES buffer (8:2, v/v, 0.02 mol/L, pH 7.4). UV–vis absorbance spectrum of approximately 43 nm for compound 23 and 46 nm for compound 24 were observed with the fluorescence quenching in solutions of 23 (U = 0.31) and 24 (U = 0.23) upon addition of Pi. Sensor 23 and 24 are the first two 1, 8-naphthyridinebased boron complexes developed, which serve as colorimetric probes capable of highly selective detection of Pi over other phosphate-based chemical species [37].
Sensor 25 was a near-infrared colorimetric and fluorescent chemosensor for detecting Pi. It exhibited a rapid response and high sensitivity towards Pi in DMSO-HEPES buffer (9:1, v/v, 0.02 mol/L, pH 7.0). A colorimetric change with a 70 nm red-shift in the UV–vis absorbance spectrum and a 72-fold enhancement in the ratio of fluorescence intensities at 656 nm and 552 nm were observed [38].
Cyanine based sensor 26 has potential to be used as a NIR functional probe for colorimetric and fluorescent Pi sensing. As predicted, the results showed that Pi promoted ester bond cleavage in compound 26, thus liberating the fluorophore, accompanied by a blue shift in the main absorption maximum from 799 nm to 430 nm and up to a 30-fold enhancement in the fluorescence intensity at 560 nm. Furthermore, all of these Pi sensors (19 - 26) were successful applied to the fluorescence in vivo imaging [39].
4. Biological neutral molecular sensors 4.1. Hydrogen sulfide sensorsWe later developed a lysosome-targeted fluorescent chemodosimeter (compound 27, Fig. 20) for monitoring endogenous and exogenous H2S by in vivo imaging of HeLa cells, D. melanogaster and C. elegans [40]. A disulfide bond was introduced to link the two coumarin fluorophores in sensor 27. The reduction of disulfide bonds to HS- was followed by the adjacent ester bonds breaking affording nucleophilic sulfhydryl groups, which cleave the neighboring ester bonds to release the fluorophore. This causes an enhancement of the fluorescence in mixed solution. Compound 27 is an excellent sensor for lysosome staining by in vivo imaging with high Pearson's and overlap coefficients. It was successfully applied to trace the accumulation of lysosomes in D. melanogaster and endogenous lysosomal injury in mutated C. elegans (SRP- 6nulls) with high resolution.

|
Download:
|
| Fig. 20. Structures of compounds 27 and 28, and the plausible reaction mechanism of 27 and 28 with H2S. | |
Due to the emission wavelength of sensor 27 being only 450 nm, investigations into better fluorescence properties of the H2S-based sensor was necessary. In 2017, Zhang et al. designed and synthesized a new cyanine-based colorimetric and fluorescence sensor (compound 28, Fig. 20) for the sensitive and selective detection of hydrogen sulfide (H2S) [41]. The reaction mechanism of sensor 28 was the same as compound 27. Upon addition of NaHS, the emission intensity of sensor 28 at 633 nm gradually increased and a red fluorescence was observed. At the same time, the color of sensor 28 changed from green to pink, which can be seen by the naked eye. In addition, sensor 28 could also be used in fluorescence imaging of living cells and C. elegans.
In 2016, Niu and coworkers designed and synthesized a novel cascade reaction-based fluorescent sensor (compound 29, Fig. 21) for the detection of H2S [42]. Unlike other published work, the disulfide bond was not introduced into the sensor 29. A fluorophore, 2-(20-hydroxy-30-methoxyphenyl)benzothiazole (HMBT), was directly connected to the 2-(bromodomethyl)benzoic acid with an ester bond. The addition of NaHS, resulted in the nucleophilic attack of HS- towards bromomethyl group generating a thiol intermediate, which is followed by a cyclization cascade reaction towards the adjacent ester carbonyl to release the fluorophore. As a consequence, an intense green fluorescence was observed. Beyond that, sensor 29 was applied to the detection and imaging of H2S in living HeLa cells, which exhibited potential biological applications.

|
Download:
|
| Fig. 21. Structure of compound 29 and the proposed sensing mechanism of compound 29 for H2S. | |
4.2. Nitroxyl sensor
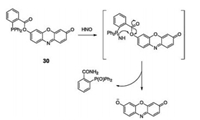
In 2015, we developed a cellular responsive, highly selective fluorogenic and chromogenic chemodosimeter sensor (compound 30, Fig. 22)for HNO detection, providing bright images against darkbackground without interference by other analytes [43]. Triphenylphosphine was introduced to the resorufin fluorophore to generate sensor 30. With the addition of HNO, sensor 30 released resorufin fluorophores caused by the reaction between HNO and the phosphine. Sensor 30 exhibited high selectivity and extreme sensitivity towards HNO at concentrations as low as 0.02 μmol/L, with a 30-fold fluorescence enhancement and a color change from pale yellow to pink. Furthermore, sensor 30 was successfully applied to trace the concentration of HNO in living systems by fluorescence imaging of hamster ovary cells and C. elegans.
|
Download:
|
| Fig. 22. Chemical structure of compound 30 and the reaction between compound 30 and HNO. | |
4.3. Nucleotide sensors
In 2014, a series of 1, 8-naphthalimide-based analogs were developed for sensing GMP, TMP, and UMP [44], which generated a significant fluorescence response with a fluorescence enhancement of more than 600-fold. The reaction mixture produced a 109 nm red-shift and a color change from colorless to brown. In the case of the NH groups of sensor 31 (Fig. 23), multiple hydrogen bonds formed with related base groups in the nucleotides. Furthermore, deprotonation of these NH groups was responsible for the unique response of sensor 31 in nucleotide sensing. The binding patterns of sensor 31 with GMP, UMP, and TMP were confirmed by theoretical calculations. Moreover, due to excellent spectroscopic properties of sensor 31, a series of biological tests were successfully conducted, illustrating its applicability in the fluorescence imaging of GMP, TMP, and UMP in C. elegans.

|
Download:
|
| Fig. 23. Chemical structure of compound 31 and the proposed binding mechanism of compound 31 with UMP, TMP and GMP. | |
Throughout the literature, Zn2+ complexes are commonly utilized as coordination sites in UDP recognition studies, while other metal complexes and ligand based nucleoside sensors have rarely been reported. Zhou and coworkers expanded this work by creating 8- naphtylimide-based sensor 32 (Fig. 24) for the fluorescence sensing of uridine diphosphate (UDP) [45]. The introduction of a 2- (propylamino)acetamide moiety to compound 32, provided enhanced binding sites for the formation of stable H-bonds with uracil's base group in UDP. This resulted in high fluorescent selectivity with a 13-fold enhancement in fluorescence intensity. Furthermore, sensor 32 displayed good cell permeability and was successfully used to detect UDP accumulation in HeLa cells and C. elegans with fluorescence imaging.

|
Download:
|
| Fig. 24. Structure of sensor 32 and the proposed binding mechanism of compound 32 with UDP. | |
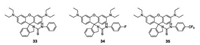
Adenosine triphosphate (ATP) is a complex organic chemical that participates in many metabolic processes. Hence, the development of novel probes for accurate and rapid sensing of ATP is vital. In 2018, Yoonand coworkersdesigned and synthesized new colorimetric and fluorescence "turn-on" sensors (33—35, Fig. 25), based on rhodamine for ATP detection [46]. Sensors 33—35 exhibited expected color and fluorescence changes via hydrogen bond interactions with ATP. Yoon found that sensor 33 projected the most desirable properties for ATP response. The addition of only 5 mmol/L ATP produced a fluorescence enhancement, of < 65-fold, with a bright red emission and a solution color change from colorless to pink. Furthermore, sensor 34 was successfully applied to fluorescence imaging in the real-time detection of ATP concentration in HeLa cells, which showed its potential application in biosystems.
|
Download:
|
| Fig. 25. Chemical structures of compounds 33—35. | |
Reports examining nucleotide probes have shown that the majority of them respond to nucleotides by intermolecular hydrogen bond interactions. Zhang et al. develope reversible rhodamine-based fluorescent sensor (36, Fig. 26), which exhibited high sensitivity and selectivity for Zn2+ and ATP [47]. A rare examination of the mechanism for ATP sensing using sensor 36 was accomplished. It stated that upon the addition of Zn2+, probe 36 bonded with Zn2+ to form complex 36-Zn2+, which contributed to an enhancement of green fluorescent and a color change from blue to colorless. However, treatment of complex 36- Zn2+ with ATP, incurred zinc expulsion, resulting in fluorescent reduction and a color change from colorless to blue. Therefore, the mechanism of ATP response is based on zinc complex ensemble displacement. In addition, sensor 36 was successfully applied to fluorescence imaging in living cells, highlighting its potential in environmental and biosystem applications.
|
Download:
|
| Fig. 26. Structure of sensor 36 and the Zn2+-ATP-sensing mechanism of sensor 36. | |
4.4. Thiol sensors
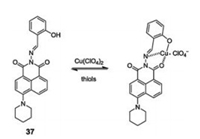
In 2013, Zhang and Zhou designed and synthesized 1, 8- naphthalimide-Cu(Ⅱ) (37-Cu) based on the "chemosensing en- semble" method for sensing thiols (Cys, Hcy, GSH) over other α- amino acids at pH 7.4 in organic aqueous media EtOH-HEPES (9:1, v/v). The synthesis of sensor 37-Cu(Ⅱ) involved the addition a speci fic volume of Cu2+ to compound 37 (Fig. 27) [48]. A noticeable color change, from colorless to pale yellow, with fluorescence quenching was observed due to the binding constant of compound 37 and Cu2+ being smaller than thiol and 37-Cu(Ⅱ). After the addition of thiols (Cys, Hcy, GSH), Cu(Ⅱ) was expelled from sensor 37-Cu(Ⅱ), resulting in a strong green-yellow florescence, caused by ligand 37. Furthermore, sensor 37-Cu(Ⅱ) was applied to thiol detection in CHO cells with high sensitivity and selectivity.
|
Download:
|
| Fig. 27. Structure of compound 37 and proposed "off-on" sensing mechanism. | |
In 2014, Yoon's group prepared two cyanine-based nearinfrared fluorescent sensors 38 and 39 (Fig. 28), which contained sulfonamide groups [49]. Sensor 38, which possessed a 2, 4- dinitrobenzenesulfonamide group, was used to detect thiol-based amino acids GSH, Cys, and Hcy in living cells. However, sensor 39, bearing a 5-(dimethylamino)naphthalenesulfonamide group, displayed unique selectivity toward GSH over Cys and Hcy. Hence it was employed in GSH detection in living cells, not only because of its selectivity but also its good cell-membrane permeability. Using mice, sensor 39 (50 μmol/L) in a buffer solution was injected intravenously, emanating strong fluorescence from the mouse's body. This study showed that sensor 39 can serve as an effective tool in the analysis of cellular GSH functions and be used to monitor GSH levels in mouse by fluorescence changes.
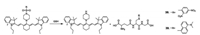
|
Download:
|
| Fig. 28. Structures of sensors 38, 39 and the proposed sensing mechanism of 38, 39 with GSH. | |
In 2015, Kim's group synthesized a Cys-speci fic two-photon fluorescent sensor (40, Fig. 29) by the incorporation of an acrylate moiety into chromene [50]. The reaction mechanism of sensor 40 is based on nucleophilic addition of Cys to the acrylate, followed by a rapid intramolecular cyclization and the release of the free chromene fluorophore. This results in colorimetric changes associated with a 46 nm red-shift in the UV–vis absorption spectrum and a 22-fold enhancement in the ratio of fluorescence intensities at 509 nm. Sensor 40 can monitor intracellular Cys alterations in different living carcinoma cells and in the case of cryosectioned tumor tissue up to a depth of 60 μm, its deep tissue penetration and low photo-cytotoxicity made it useful in various biological and clinical applications.

|
Download:
|
| Fig. 29. Chemical structure of sensor 40 andthe Cys-sensing mechanism ofsensor 40. | |
In the sensing of thiols Cys, Hcy or GSH reports have described numerous probes capable of high selectivity. In 2017, Zhou's group designed and synthesized a coumarin-based "turn-on" fluorescent sensor (41, Fig. 30) for Cys detection [51]. The reaction mechanism was attributed to the cysteine-induced SNAr substitution-rear-rangement reaction. Withan increasein thiol concentration, caused by the addition of Cys, chloro substitution is promoted, followed by rearrangement resulting in the formation of a 7-membered macrocyclic state and a strong green fluorescence. In addition, sensor 41 was successfully applied to fluorescence imaging for the purpose of cysteine detection in Hi5 cells and C elegans.

|
Download:
|
| Fig. 30. Chemical structure of compound 41 and the proposed binding mechanism of compound 41 with Cys. | |
5. Sensors of tumor microenvironment
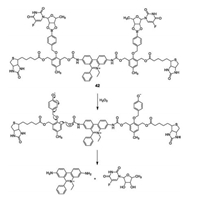
In 2014, Jong Seung Kim and coworkers reported a mitochondria-targeting antitumor theranostic agents (theranostic 42, Fig. 31), which contained two molecules of the active drug 50-deoxy-5- fluorouridine and an apoptotic marker ethidium [52]. The prodrug can selectively detect endogenous mitochondrial-overexpressed H2O2 and release potent anticancer drug 50-deoxy-5-fluorouridine and apoptotic marker ethidium in the tumor cells. The red fluorescence of ethidium can be utilized to monitor the in vitro and in vivo release of the drug. After drug activation in the mitochondria, theranostic 42 induced intrinsic apoptosis in tumor cells followed by the activation of genomic DNA or RNA damage pathways. A remarkable suppression of tumor growth was observed after treatment with theranostic 42 in a xenograft mouse model. The fluorescence intensity of tumor issue was increased 7-fold than the normal one. Compared with 5-fluorouracil, theranostic 42 displayed enhanced cytotoxicity (20% enhance). Moreover, theranostic 42 is the first mitochondria-targeting antitumor theranostic agent ever reported, upon activation it permits a self-monitoring apoptosis of cancer cells leading to precise cancer treatment.
|
Download:
|
| Fig. 31. Structure and mechanism of theranostic 42 and fluorophore activation after reaction with H2O2. | |
During this time the Jong Seung Kim group also synthesized theranostic agent 43 (Fig. 32) in which coum4a3rin was used as the fluorophore and boronate ester was used as a trigger unit [53]. The addition of H2O2 activated the fluorophore, releasing therapeutic drug SN-38, which is an analogue of camptothecin (CPT). The entire process was monitored via the gradual increase in the intensity of blue fluorescence. Prodrug 43 not only showed that the fluorescent signal was localized inside the cell, but also the selective inhibition of tumor cell growth. In addition, the intratracheal administration of prodrug 43 showed effective antitumor activity in a mouse model with metastatic lung disease. After comparing with the mice tissue which was treated with saline, histological sections of lung tissues revealed that cellular cavitation was alleviative. Thus, H2O2- responsive prodrug 43 possesses a therapeutic potential for selective accumulation in lung tumors and a signi ficant antineplastic activity in metastatic lung disease of mice.

|
Download:
|
| Fig. 32. Chemical structure of compound 43 and the reaction between compound 43 and H2O2. | |
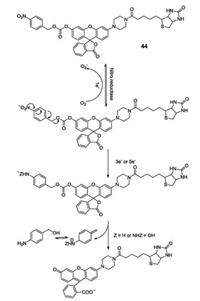
Nitroreductases (NTRs) are a family of flavin-containing enzymes that catalyze the reduction of nitroaromatic compounds to the corresponding amines using either NADH or NADPH as a source of reducing equivalents. As nitroreductases over express in hypoxic conditions in solid tumors, it is important to develop highly selective fluorescent NTR-targeted probes, which are sensitive to the hypoxia micro-environment. Zhou and coworkers designed and synthesized "turn-on" nitroreductase sensor 44 (Fig. 33), which contains biotinylated-piperazine-rhodol in conjunctionwith an NTR responsive self-immolative p-nitrobenzyl moiety via a carbonate linker [54]. Upon addition of NTRs, the ester bonds of sensor 44 were cleaved, releasing biotinylated-piperazine-rhodol and emitting a strong green fluorescence from fluorophore rhodol. Moreover, sensor 44 was successfully used to sense NTRs in Hi5 cells and C. elegans in fluorescence imaging studies.
|
Download:
|
| Fig. 33. Chemical structure of sensor 44 and the reaction between probe 44 and NTRs. | |
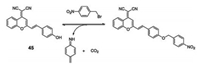
In solid tumors, expression of NTRs is directly related tothe hypoxic status. Nowadays, the common goal in such studies was to measure the hypoxia in tumor cells or tumor-derived tissue utilizing a sensitive fluorescence sensor. Therefore, Zhou's group developed elective fluorescent nitroreductase sensor 45 (Fig. 34), composed of a typical donor-π-acceptor featured chromophore, dicyanomethylene-4H- pyran (DCM), and a nitrobenzene group, to sensitively monitor the NTR level [55]. The elongated conjugated system and asymmetric structure resulted in a push-pull construction that allowed the design of the sensor to have a stronger fluorescence signal, and shorter emission wavelength in the presence of NTR, when compared to the control conditions in which NTR is not present. Cleavageof the O-C bond at the terminal ends of NTR, promotesthe release of the chromophore and a green fluorescence. Furthermore, due to the excellent cell permeability of sensor 45, it was used for detecting exogenous NTR in C. elegans, and the hypoxic status in Hi5 cells. Moreover, in mice bearing HEPG-2 induced tumors, after 5 h of incubation with 100 μmol/L of sensor 45 at 37 ℃ under the hypoxic condition, tumor tissue displayed a strong fluorescence signal when compared to the surrounding tissues. This exhibited that sensor 45 is a practical sensor for sensing hypoxic condition in tumor tissue.
|
Download:
|
| Fig. 34. Structure of sensor 45 and the proposed sensing mechanism of 45 with NTRs. | |
In 2016, the Jong Seung Kim group undertook a theranostic study in which azobenzene scaffold served as both fluorescent quenchers and nitrogen mustard deactivators in the mitochondrial targeting units bearing therapeutic drug delivery systems (DDS, sensor 46, Fig. 35) [56]. It exhibited tissue selectivity for tumors with aggressive phenotypes and the ef ficient in vitro and in vivo azoreduction under the hypoxic condition. In the cell experiments, the fluorescence enhancement of sensor 46 at 557 nm in hypoxic tumor cells was observed, but no signi ficant fluorescence change in normal cells or normoxic tumor cells was found. Furthermore, the tumor xenograft murine model shows that sensor 46 emits a clear fluorescence in the tumor area and the release of N, N-bis(2- chloroethyl)-1, 4-benzenediamine caused a rapid decrease in the size and weight of the tumors. Mechanistic insight of the DDS's mode of action was gained by gene and protein expression experiments, which proved that the treatment was the result of mitochondrially triggered apoptosis via the granzyme A and B signaling pathways and resulted in tissue normalization.

|
Download:
|
| Fig. 35. Chemical structure of compound 46 and the proposed mode of action of compound 46. | |
In 2018, the Jong Seung Kim group reported prodrug 47 (Fig. 36), this time for the diagnosis and treatment of colon cancer. They discovered that prodrug 47 was preferentially taken up by colon cancer cells through receptor-mediated endocytosis, whilst simultaneously releasing the active drug Dox with a fluorescence enhancement at 590 nm after activation via lysosomal β-Gal enzyme in cancer cells [57]. In order to demonstrate the speci fic recognition of the probe for colon cancer cells with ASGP receptor- overexpressing, a control experiment was performed. Sensor 47 showed signi ficant intracellular fluorescence both in HT-29 cells, a colon adenocarcinoma cell line, and HepG2 cells, a common cancer cell line carrying the ASGP receptor, while a relatively low fluorescence signal was detected in HeLa cells. In order to evaluate the anti-tumor activity of sensor 47, the targeting ability of the probe to tumor in vivo was first evaluated by using an in vivo fluorescence imaging system, saline, free Dox and Gal-Dox (sensor 47). A single dose of 3 mg/kg was intravenously injected into the mouse carrying the HT-29 tumor, and the whole-body fluorescence imaging result after 2 days showed that the probe had excellent targeting ability to tumors. Afterwards, the in vivo therapeutic effect of the probe was evaluated in mice bearing HT-29 tumors, and the inhibition rate of sensor 47 to the tumor reached 53.1% while the free Dox was 34.9%. Hence, sensor 47 may possibly have potential to be an ideal theranostic system in future personalized cancer chemotherapeutics.

|
Download:
|
| Fig. 36. Chemical structure and activation mode for compound 47. | |
In 2017, Yoon's group reported a near-infrared fluorescent probe of two-part cyanine analogs linked by a peptide chain, which can be cleaved by cathepsin B [58]. After the peptide bond is cleaved, the FRET process is blocked, with the two cleaved cyanine parts fluoresce at 720 nm and 780 nm, respectively. Furthermore, sensor 48 (Fig. 37) showed speci fic phototoxicity to tumor cells, due to the activity of cathepsin B in tumor cells being much higher than in normal cells. The final cleavage product of the probe is a PDT reagent that exhibits strong phototoxicity to tumors at 808 nm laser irradiation. Cathepsin B is mainly located in the lysosomes of tumor cells. During the imaging of probes in HeLa cell lysosomes, the increase and spread fluorescence in the cytoplasm after lysosomal membrane destruction can be observed. This indicates that sensor 48 can locate cathepsins and monitor the lysosomal membrane permeabilization (LMP) process. In order to expand the imaging application of sensor 48 in vivo and evaluate its anti-tumor ef ficacy, the probe was intravenously injected into tumor mice, and a strong fluorescence was observed in the tumor area after 4 h and maintained for 32 h. In the control experiment the tumor volume in mice irradiated with the 808 nm laser was reduced by one-third after 14 days. These results endorse CyA-P-CyB as a truly ef ficient phototherapy reagent.
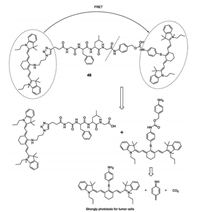
|
Download:
|
| Fig. 37. Structure of sensor 48 and how 48 is triggered by cathepsin B. | |
6. Conclusion
In this review, we present the development and application of fluorescent and colorimetric sensors for ion sensing, biologically neutral molecular-recognition and tumor micro-environment-related parameters sensing. The sensors were organized into categories in accordance with their target species, such as cation and anion sensors, as well as nucleotide, thiol and tumor micro-environment-related sensors. The bene fit of low toxicity possessed by fluorescence sensors enables them to be utilized in live animal models, allowing for a broader application of these chemical tools in biomedical research. Application of fluorescence imaging technologies helps in the elucidation of the mechanism, the reactive species signaling pathways, the cellular condition, and the disease states with a simple and rapid response. Theranostics, afusion ofspecialized diagnosis and therapy, is an emerging area of drug discovery in recent years. The rational design of fluorescent prodrug conjugates, that are not only selectively recognized and internalized by speci fic tumor cells, but also undergo stimulus-promoted cleavage to produce both a fluorogenic response and release an active cytotoxic drug, are therefore attractive in present and future sensor research fields. It is our hope that this review will provide an overview of seminal and current research efforts that will stimulate the interest of scientists leading to the development of new sensing approaches, and producing effective and economically attractive sensor systems.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21462050 and 21672185), the Foundation of the Department of Science and Technology of Yunnan Province of China (No. 2016FB020), and the Program for Excellent Youth Talents (No. C6176102), Yunnan University.
| [1] |
V.S. Lin, W. Chen, M. Xian, C.J. Chang, Chem. Soc. Rev. 44 (2015) 4596-4618. DOI:10.1039/C4CS00298A |
| [2] |
M.H. Lee, J.S. Kim, J.L. Sessler, Chem. Soc. Rev. 44 (2015) 4185-4191. DOI:10.1039/C4CS00280F |
| [3] |
Y. Zhou, J. Yoon, Chem. Soc. Rev. 41 (2012) 52-67. DOI:10.1039/C1CS15159B |
| [4] |
T.D. Ashton, K.A. Jolliffe, F.M. Pfeffer, Chem. Soc. Rev. 44 (2015) 4547-4595. DOI:10.1039/C4CS00372A |
| [5] |
Z.G. Yang, J.F. Cao, X.J. Peng, et al., Chem. Soc. Rev. 43 (2014) 4563-4601. DOI:10.1039/C4CS00051J |
| [6] |
J.J. Du, M.M. Hu, J.L. Fang, X.J. Peng, Chem. Soc. Rev. 41 (2012) 4511-4535. DOI:10.1039/c2cs00004k |
| [7] |
Y. Zhou, J.F. Zhang, J. Yoon, Chem. Rev. 114 (2014) 5511-5571. DOI:10.1021/cr400352m |
| [8] |
J.F. Zhang, Y. Zhou, J. Yoon, J.S. Kim, Chem. Soc. Rev. 40 (2011) 3416-3429. DOI:10.1039/c1cs15028f |
| [9] |
H.N. Kim, W.X. Ren, J.S. Kim, J. Yoon, Chem. Soc. Rev. 41 (2012) 3210-3244. DOI:10.1039/C1CS15245A |
| [10] |
E. Climent, A. Agostini, Santos-Figueroa L.E., et al., Chem. Soc. Rev. 42 (2013) 3489-3613. DOI:10.1039/c3cs35429f |
| [11] |
Y. Zhou, Z.C. Xu, J. Yoon, Chem. Soc. Rev. 40 (2011) 2222-2235. DOI:10.1039/c0cs00169d |
| [12] |
L.Y. Niu, Y.Z. Chen, H.R. Zheng, et al., Chem. Soc. Rev. 44 (2015) 6143-6160. DOI:10.1039/C5CS00152H |
| [13] |
X.Q. Chen, Y. Zhou, X.J. Peng, J. Yoon, Chem. Soc. Rev. 39 (2010) 2120-2135. DOI:10.1039/b925092a |
| [14] |
M. Gao, F.B. Yu, C.J. Lv, et al., Chem. Soc. Rev. 46 (2017) 2237-2271. DOI:10.1039/C6CS00908E |
| [15] |
M.H. Lee, A. Sharma, J.M. Chang, et al., Chem. Soc. Rev. 47 (2018) 28-52. DOI:10.1039/C7CS00557A |
| [16] |
Y. Zhou, Y. Xiao, X.H. Qian, Tetrahedron Lett. 49 (2008) 3380-3384. DOI:10.1016/j.tetlet.2008.03.128 |
| [17] |
Y. Zhou, F. Wang, Y.M. Kim, J.Y. Yoon, Org. Lett. 11 (2009) 4442-4445. DOI:10.1021/ol901804n |
| [18] |
J.F. Zhang, Y. Zhou, J.Y. Yoon, et al., Org. Lett. 12 (2010) 3852-3855. DOI:10.1021/ol101535s |
| [19] |
Y.L. Duan, G.K. Wang, Y. Zhou, et al., Tetrahedron Lett. 53 (2012) 6544-6547. DOI:10.1016/j.tetlet.2012.09.089 |
| [20] |
Y. Zhou, H.N. Kim, J.Y. Yoon, Bioorg. Med. Chem. Lett. 20 (2010) 125-128. DOI:10.1016/j.bmcl.2009.11.028 |
| [21] |
L.Y. Zhao, J.F. Zhang, Q.H. Zhao, et al., Tetrahedron Lett. 54 (2013) 3353-3358. DOI:10.1016/j.tetlet.2013.04.045 |
| [22] |
Y.G. Shi, J.H. Chen, Y. Zhou, et al., Bull. Korean Chem. Soc. 34 (2013) 63-67. DOI:10.5012/bkcs.2013.34.1.63 |
| [23] |
G.K. Wang, B. Liu, J.F. Zhang, et al., Chem. Asian J. 9 (2014) 744-748. DOI:10.1002/asia.v9.3 |
| [24] |
X.M. Li, R.R. Zhao, J.F. Zhang, et al., Chin. Chem. Lett. 27 (2016) 813-816. DOI:10.1016/j.cclet.2016.04.001 |
| [25] |
L.E. Guo, H. Wang, J.F. Zhang, et al., Chem. Asian J. 10 (2015) 1898-1902. DOI:10.1002/asia.v10.9 |
| [26] |
X.M. Li, R.R. Zhao, J.F. Zhang, et al., Chin. Chem. Lett. 28 (2017) 1258-1261. DOI:10.1016/j.cclet.2016.12.029 |
| [27] |
Y. Zhang, Y. Fang, G.Z. Wu, et al., Chin. Chem. Lett. 27 (2016) 1673-1678. DOI:10.1016/j.cclet.2016.04.011 |
| [28] |
Q. Wang, J. Jiang, Z.M. Zhou, et al., Chin. Chem. Lett. 27 (2016) 1563-1566. DOI:10.1016/j.cclet.2016.02.021 |
| [29] |
Y. Long, J. Zhou, M.P. Yang, B.Q. Yang, Chin. Chem. Lett. 27 (2016) 205-210. DOI:10.1016/j.cclet.2015.09.003 |
| [30] |
Y. Zhou, J.Y. Jung, J.Y. Yoon, et al., Org. Lett. 13 (2011) 2742-2745. DOI:10.1021/ol200846a |
| [31] |
L.Y. Zhao, B. Liu, J.F. Zhang, et al., J. Fluorine Chem. 158 (2014) 53-59. DOI:10.1016/j.jfluchem.2013.11.002 |
| [32] |
X.Y. Wang, B. Li, H. Zhang, et al., Chin. Chem. Lett. 27 (2016) 211-214. DOI:10.1016/j.cclet.2015.11.014 |
| [33] |
R.R. Zhao, Q.L. Xu, J.F. Zhang, et al., Tetrahedron Lett. 57 (2016) 5022-5025. DOI:10.1016/j.tetlet.2016.09.081 |
| [34] |
L.E. Guo, J.F. Zhang, Y. Zhou, et al., Anal. Chem. 87 (2015) 1196-1201. DOI:10.1021/ac503818p |
| [35] |
J.F. Zhang, L.E. Guo, Y. Zhou, et al., Chem. Asian J. 10 (2015) 1165-1169. DOI:10.1002/asia.v10.5 |
| [36] |
H. Wang, L.E. Guo, J.F. Zhang, et al., Dyes Pigments 120 (2015) 293-298. DOI:10.1016/j.dyepig.2015.04.031 |
| [37] |
G.F. Wu, L.E. Guo, J.F. Zhang, et al., Tetrahedron Lett. 56 (2015) 5034-5038. DOI:10.1016/j.tetlet.2015.07.025 |
| [38] |
T.N. Zang, B. Liu, J.F. Zhang, et al., RSC Adv. 5 (2015) 71756-71759. DOI:10.1039/C5RA12835H |
| [39] |
B. Liu, D. Yang, J.F. Zhang, et al., Dyes Pigments 133 (2016) 127-131. DOI:10.1016/j.dyepig.2016.04.032 |
| [40] |
X.J. Zou, Y.C. Ma, C.G. Zhou, et al., Chem. Commun. 50 (2014) 13833-13836. DOI:10.1039/C4CC05539J |
| [41] |
H. Wang, D. Yang, J.F. Zhang, et al., Sens. Actuators B 247 (2017) 883-888. DOI:10.1016/j.snb.2017.03.030 |
| [42] |
H.R. Zheng, Y.Z. Chen, Q.Z. Yang, et al., Chin. Chem. Lett. 27 (2016) 1793-1796. DOI:10.1016/j.cclet.2016.04.023 |
| [43] |
K.N. Bobba, Y. Zhou, J.F. Zhang, et al., RSC Adv. 5 (2015) 84543-84546. DOI:10.1039/C5RA17837A |
| [44] |
L.M. Zhang, X.M. Li, J.F. Zhang, et al., Tetrahedron Lett. 55 (2014) 6131-6136. DOI:10.1016/j.tetlet.2014.09.074 |
| [45] |
X.W. Lv, G.K. Wang, X.G. Xie, et al., Dyes Pigments 142 (2017) 552-557. DOI:10.1016/j.dyepig.2017.04.006 |
| [46] |
Y.F. Liu, D. Lee, D. Wu, et al., Sens. Actuators B 265 (2018) 429-434. DOI:10.1016/j.snb.2018.03.081 |
| [47] |
X.L. Jin, X.L. Wu, B. Wang, et al., Sens. Actuators B 261 (2018) 127-134. DOI:10.1016/j.snb.2018.01.116 |
| [48] |
Y.G. Shi, J.H. Yao, Y. Zhou, et al., Bioorg. Med. Chem. Lett. 23 (2013) 2538-2542. DOI:10.1016/j.bmcl.2013.03.004 |
| [49] |
J. Yin, Y. Kwon, D. Kim, et al., J. Am. Chem. Soc. 136 (2014) 5351-5358. DOI:10.1021/ja412628z |
| [50] |
Y.H. Lee, W.X. Ren, J. Han, et al., Chem. Commun. 51 (2015) 14401-14404. DOI:10.1039/C5CC06038A |
| [51] |
Y. Yang, H. Wang, J.F. Zhang, et al., Chin. Chem. Lett. 28 (2017) 2023-2026. DOI:10.1016/j.cclet.2017.08.051 |
| [52] |
R. Kumar, J. Han, H.J. Lim, et al., J. Am. Chem. Soc. 136 (2014) 17836-17843. DOI:10.1021/ja510421q |
| [53] |
E.J. Kim, S. Bhuniya, H. Lee, et al., J. Am. Chem. Soc. 136 (2014) 13888-13894. DOI:10.1021/ja5077684 |
| [54] |
X.W. Lv, D. Yang, J.F. Zhang, et al., Analyst 142 (2017) 345-350. DOI:10.1039/C6AN02107G |
| [55] |
D. Yang, H.Y. Tian, M. Li, Y. Zhou, Sci. Rep. 7 (2017) 9174-9182. DOI:10.1038/s41598-017-09525-2 |
| [56] |
P. Verwilst, J. Han, J. Lee, et al., Biomaterials 115 (2016) 104-114. |
| [57] |
A. Sharma, E.J. Kim, H. Shi, et al., Biomaterials 155 (2018) 145-151. DOI:10.1016/j.biomaterials.2017.11.019 |
| [58] |
X.Q. Chen, D.Y. Lee, S.S. Yu, et al., Biomaterials 122 (2017) 130-140. DOI:10.1016/j.biomaterials.2017.01.020 |
 2018, Vol. 29
2018, Vol. 29 


