b Key Laboratory of Theoretical and Computational Photochemistry, Ministry of Education, College of Chemistry, Beijing Normal University, Beijing 100875, China
During the past few decades, a great growth of research interest has occurred in the fields of the asymmetric synthesis and separation [1-3]. The aim of enantio selective synthesis or catalysis is to obtain chiral products i.e., a pure enantiomer as the ultimate goal) starting from achiral substrates or in the presence of chiral reagents. Actually, the final products are usually more complex than the designed systems. For example, both the reactant and the product can exist as a mixture of conformational isomers, and several conformations can be largely populated, and they can also exist in different states of aggregation or solvation [4]. So how to obtain stereoselectivity remains to be a challenge.
Cocrystals generally stand for the orderly molecular solids composed of two or more types of neutral chemical species [5]. Recently, cocrystals have been paid much attention due to the ability to modify the physical and chemical properties of organic solid-state molecular materials [6-10]. As a result, cocrystal formation has been effectively utilized in the fields of pharmaceutics [11], luminescence [12] and optoelectronic devices [13]. Theoretically, the chiral cocrystals can be facilely designed based on the self-assembly of chiral molecules. However, to date, the chiral cocrystals are still relative limited [14], and how the occurrence of molecular recognition between the chiral and achiral molecules is still not fully understood.
2, 2'-Binaphthol (BINOL) and its derivatives have generated particular interest because their versatile backbone for asymmetric synthesis. The original synthesis of BINOL, reported by Pummerer et al. in 1926 [15], involves facile oxidative coupling of the two naphthol units induced by FeCl3. Since then, a wide range of other coupling methods for the preparation of racemic BINOL have been developed [4]. However, until 1979, the asymmetric product was found by Noyori in the reduction of aromatic ketones [16]. BINOL is well known to racemize under acidic or basic conditions, so how to obtain pure achiral BINOL compound and/or how to change the configuration of BINOL still need to be further detected and understood. Moreover, although it was documented that the enantiomerically pure BINOL can be prepared, how to turn a racemic mixture into a single chiral material still need more research.
In this work, based on a cocrystallization process of enantiomerically chiral BINOL S. and R.) with the suitable co-assembled unit, 100% R configuration of BINOL can be obtained within the cocrystal products. The high-quality R-BINOL cocrystal sample is easy to obtain through a facile solvent evaporation. 2-(3-Pyridyl)-1H-benzimidazole (namely as P.) was chosen as the co-assembled unit, because it can potentially form a co-crystal with racemic BINOL due to the supramolecular interactions between P. and BINOL (Scheme 1). The BINOL in co-crystal has been proved to be pure R (named P.R.) absolute configuration by single crystal X-ray diffraction and circular dichroism (CD) spectra. The results show that no matter what the ratio between R and S is, all S configuration of BINOL will be changed to be R configuration. Additionally, the detailed Raman/fluorescence emission spectra and fluorescence lifetime of the cocrystal were also determined. Therefore, this work gives an investigation on the transformation of the chiral configuration based on co-crystallization, which may be potentially used into chiral luminescent imaging fields.

|
Download:
|
| Scheme 1. Molecular structure of different configurations (S or R) of BINOL and 2-(3-pyridyl)-1H-benzimidazole (P.) | |
Analytically pure 2, 2'-binaphthol, 2-(3-pyridyl)-1H-benzimidazole and methanol were purchased from J & K Scientific Ltd. and used without further purification. The deionized and decarbonated water was used in the preparation process.
Typically, the co-crystal was prepared by a slow evaporation of a methanol solution containing P. and BINOL with a 1:1 stoichiometry. Typically, 0.5 mmol of 2, 2'-binaphthol and 0.5 mmol of 2-(3- pyridyl)-1H-benzimidazole were dissolved in 20 mL of methanol, and the solution was then put in a fuming cupboard for 7 days.
X-ray diffraction (XRD) patterns of samples were recorded using a Rigaku2500VB2 + PC diffractometer under the following conditions: 40 kV, 50 mA, Cu Kα radiation (λ = 0.154 056 nm) with step scanning of 0.04° (2θ) in the range 3°–20° using account time of 10 s/step. The solid-state fluorescence spectra were recorded on an RF-5301PC fluorospectrophotometer, with the widths of both the excitation and emission slits being 3 nm. The fluorescence images were obtained with a Nikon A1R-si laser scanning confocal microscope, and a galvano scanner was used to capture highquality 32-channel spectral images. The fluorescence decays were measured using a LifeSpec-ps spectrometer, and the lifetimes were calculated with the F900 Edinburgh instruments software. The Raman spectra were obtained with a Bruker VERTEX 7v vacuum Raman spectrometer. The circular dichroism spectra were recorded using a JASCO J-815 circular dichroism spectrometer.
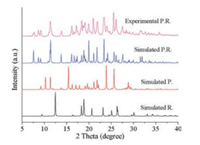
The co-crystal was synthesized by the slow evaporation of a methanol solution containing P. and BINOL (R. or S.) with a 1:1 stoichiometry. The powder X-ray diffraction (XRD) pattern for the as-obtained co-crystal is shown in Fig. 1. In each case, the reflections fit well with the simulated structure based on the absolute configuration of P.R., confirming the high purity of the sample.
|
Download:
|
| Fig. 1. Comparison of experimental XRD patterns of P.R. with the simulated result based on the single-crystal structure | |
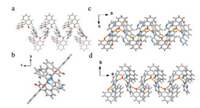
Absolute configuration of the cocrystals shows that all final products present the same structure as the P.R., no matter the molecular assembly unit is R or S BINOL. This suggests that there is a conformational transfer from BINOL (S) to BINOL (R) during the cocrystallization process. Fig. a shows the assembly modes of P.R., which confirms that the P.R. crystalline solids are assembled via the expected hydrogen bonding between the naphthol group in BINOL and the N atoms of benzimidazole unit in the co-formers. Different perspectives of the stacking fashions (Figs. 2b–d) indicate that there is a ladder structure between BINOL and P. Such stacking results in a strong π-π interaction, which facilitates the formation of P.R. More detailed crystal information can be found in Table S1 (Supporting information).
|
Download:
|
| Fig. 2. Assembly modes (a) and stacking fashions from different directions (b–d) of P.R | |
Fluorescence spectra of the P.R. sample present the two major emission peaks located at ca. 358 nm and 373 nm, which exhibits the emission wavelength from R. (at ca. 357 nm and 368 nm). The single crystals of the P.R. sample exhibited a faint yellow color and purple-light emission (λem(max) = 373 nm, Fig. S1 in Supporting information) under daylight and UV light, respectively (Fig. 3). To understand the excitation states of the cocrystals, fluorescence decay curves (Fig. 4) were performed, which show that pristine solid R-/S-BINOL have similar excited state properties with fluorescence lifetimes of 2.71 ns and 3.01 ns (Fig. 4a), respectively. The co-crystal P.R. has a shorter lifetime of 1.4 ns (Fig. 4b).

|
Download:
|
| Fig. 3. Photographs of cocrystal of P.R. under daylight (a), and UV irradiation (b), cocrystal of P.S. under daylight (c), and UV irradiation (d) observed using a fluorescence microscope | |

|
Download:
|
| Fig. 4. Fluorescence decay curves for (a) pure BINOL, (b) co-crystal remove from solvent after 10 min and 10 days | |
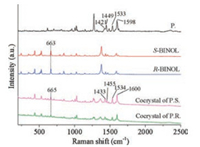
Raman spectroscopy was employed to characterize the molecular vibrations within the pure BINOL and the co-crystal due to the highly symmetric structure of BINOL (Fig. 5). For the pristine P., the characteristic C=N stretching vibration bands of imidazole group appear at 1533 cm-1, and the peak located at 1421, 1449, and 1598 cm-1 can be assigned to the C=C vibration of phenyl group. Upon assembly with the BINOL, only slight change appeared in the C=N vibration bands at 1533 cm-1 towards 1534 cm-1 for P.R. While the C=C vibration bands experienced an obvious high-frequency shift and were located at 1433 cm-1 (Δ = 12 cm-1), 1455 cm-1 (Δ = 6 cm-1) and 1600 cm-1 (Δ = 2 cm-1) for P.R., respectively, indicative of the relative strong hydrogen bonding interaction between the BINOL and N atoms in the imidazole group. For BINOL, the C-O vibration bands underwent a blue-shift from 663 cm-1 to 665 cm-1 (Δ = 2 cm-1), indicating that molecular P. can polarize and delocalize the electronic density of the BINOL molecules within adjacent layer, which weakens the C-O bond in BINOL to some extent.
|
Download:
|
| Fig. 5. Raman spectroscopy for pure R-BINOL, S-BINOL, molecular P. and their co-crystal. | |
To better understand the self-assembly process and the chiral transformation during the cocrystallization, circular dichroism spectra were performed. It was observed that both pure BINOL and BINOL@P. in solution are proved to maintain the initial chiral properties, and the S-BINOL@P. presents an opposite circular dichroism trend relative to those of the R-BINOL@P. in solution as shown in Figs. 6a and b. This behavior shows that the no chiral transformation occurs in the solution states. Figs. 6c and d show that when the solid-state BINOL@P. forms, all S configuration of BINOL will change into R configuration. This conclusion can also be extended to that no matter what is the ratio of R/S, the final configuration of BINOL is R type within the cocrystal product. This may be related to the fact that the conformation transformation of S type during the molecular recognition process, and thus results in the optimized crystal structures.

|
Download:
|
| Fig. 6. Circular dichroism spectra of pure solution of R-/S-BINOL, solutions of R-/S-BINOL with P. (a, b) and co-crystal products (c and d) | |
In summary, the luminescent chiral cocrystal of 2-(3-pyridyl)- 1H-benzimidazole and 2, 2'-binaphthol can be obtained through a solution evaporation method. Both the pristine co-assembled units R-BINOL or S-BINOL give rise to the R conformation within the final cocrystal, as confirmed by both single-crystal X-ray diffraction and circular dichroism spectra. Fluorescence spectra show that the emission band are very close to those of the pristine R-BINOL or S-BINOL sample, and the fluorescence lifetime was reduced compared with the pristine BINOL. It can be anticipated that, by the appropriate choice of the chiral chromophore as molecular self-assembled unit, the chiral cocrystal can be easily obtained, and the approach can be readily extended and be applicable to other systems that have much flexibility and potential applications for the design of chiral luminescent materials.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (NSFC, Nos. 21771021 and 21473013), the National Basic Research Program of China (973 Program, No. 2014CB932103), and Beijing Municipal Natural Science Foundation No. 2152016).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.12.021.
| [1] |
R. Noyori, Chem. Soc. Rev. 18 (1989) 187-208. DOI:10.1039/cs9891800187 |
| [2] |
J. Wilting, M. Janssen, C. Muller, et al., Adv. Synth. Catal. 349 (2007) 350-356. DOI:10.1002/(ISSN)1615-4169 |
| [3] |
F.Y. Jiang, B. Liu, Z.B. Dong, et al., J. Organomet. Chem. 692 (2007) 4377-4380. DOI:10.1016/j.jorganchem.2007.07.003 |
| [4] |
J.M. Brunel, Chem. Rev. 105 (2005) 857-897. DOI:10.1021/cr040079g |
| [5] |
X. Fang, X. Yang, D. Yan, J. Mater. Chem. C 5 (2017) 1632-1637. DOI:10.1039/C6TC05048D |
| [6] |
G. Fan, D. Yan, Adv. Opt. Mater. 4 (2016) 2139-2147. DOI:10.1002/adom.v4.12 |
| [7] |
G. Fan, X. Yang, R. Liang, et al., CrystEngComm 18 (2016) 240-249. DOI:10.1039/C5CE02019K |
| [8] |
S. Li, Y. Lin, D. Yan, J. Mater. Chem. C 4 (2016) 2527-2534. DOI:10.1039/C6TC00067C |
| [9] |
D. Yan, B. Patel, A. Delori, et al., Cryst. Growth Des. 13 (2013) 333-340. |
| [10] |
D. Yan, Y. Lin, Q. Meng, et al., Cryst. Growth Des. 13 (2013) 4495-4503. DOI:10.1021/cg400979d |
| [11] |
G. Bruni, M. Maietta, V. Berbenni, et al., J. Phys. Chem. B 118 (2014) 9180-9190. DOI:10.1021/jp503256k |
| [12] |
D. Yan, H. Yang, Q. Meng, et al., Adv. Funct. Mater. 24 (2014) 587-594. DOI:10.1002/adfm.201302072 |
| [13] |
D. Yan, D.G. Evans, Mater. Horiz. 1 (2014) 46-57. DOI:10.1039/C3MH00023K |
| [14] |
O. Sánchez-Guadarrama, F. Mendoza-Navarro, A. Cedillo-Cruz, et al., Cryst. Growth Des. 16 (2016) 307-314. DOI:10.1021/acs.cgd.5b01254 |
| [15] |
R. Pummerer, E. Prell, A. Rieche, Ber. Dtsch. Chem. Ges. 59 (1926) 2159-2161. DOI:10.1002/cber.19260590881 |
| [16] |
R. Noyori, I. Tomino, Y. Tanimoto, J. Am. Chem. Soc. 101 (1979) 3129-3131. DOI:10.1021/ja00505a056 |
 2018, Vol. 29
2018, Vol. 29 


