b State Key Laboratory of Medicinal Chemical Biology, College of Pharmacy and Tianjin Key Laboratory of Molecular Drug Research, Nankai University, Tianjin 300353, China;
c Guangxi Collaborative Innovation Center of Study on Functional Ingredients of Agricultural Residues, Guangxi Key Laboratory of Efficacy Study on Chinese Materia Medica, Guangxi University of Chinese Medicine, Nanning 530200, China;
d JF-Pharmaceutical Technology Development Co., Ltd., Tianjin 300457, China
Reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), are important by-products of oxygen metabolites [1]. They are vital to life, but become very harmful in the case of depletion of antioxidants, resulting in oxidative stress through the oxidation of biomolecules [2]. It is necessary to use sensitive and selective tools to study H2O2 in the living system because of its active and transient properties [3]. The fluorescence probe is a powerful tool due to its high sensitivity, simple data acquisition in high performance liquid chromatographic (HPLC), and high spatial resolution in microscopic imaging techniques.
In recent years, numerous in vitro online antioxidant assays have been developed to measure the radical scavenging ability of natural compounds, including HPLC-UV-ABTS [4], HPLC-UV–DPPH [5], HPLC-DAD-DPPH and HPLC-DAD-ABTS [6]. In addition, the determination of peroxides by HPLC-FLD has developed gradually. For example, Guilbault proposed using horseradish peroxidase (HRP) as a catalyst and 4-hydroxyphenylacetic acid (PHPAA) as a substrate to determine peroxide [7]. Qi et al. [8] reported the catalysis of a hemin substitute for HRP in a post-column-derived HPLC system. The common feature of these methods is that all antioxidants or peroxides are separated by a chromatographic approach, but normally, data about antioxidant capacity that are generated by in vitro methods cannot be extrapolated to in vivo effects, and most in vitro chemical processes are not relevant for in vivo conditions [9].
Luckily, recent progress regarding the biochemistry of molecular probes has enabled researchers to image H2O2 in complex living systems [10-13]. For example, HyPerRed is a genetically encoded red fluorescent sensor for low concentrations of H2O2 that is produced in the cytoplasm of cultured cells after growth factor stimulation [9]. CSBOH (Changsha near-infrared dye) was used to detect H2O2 under alkaline conditions and used for H2O2 imaging in vivo [14]. MitoB ((3-hydroxybenzyl)triphenylphosphonium bromide) is a ratiometric mass spectrometry probe that is used to assess changes in H2O2 within the mitochondrial matrix of living Drosophila [15]. NBCD (N-borylbenzyloxycarbonyl-3, 7-dihydroxyphenoxazine) is a new fluorescent probe that can detect H2O2 levels both in tissues and cells; the mechanism involves the release of resorufin from NBCD, and red fluorescence is generated after a series of reactions with H2O2 [16].
Furthermore, biological experiments are necessary to explore the antioxidant properties of compounds, which assist the microinjector system combined with a fluorescent probe microinjection technique to demonstrating antioxidant activity in vivo. Drosophila melanogaster has been extensively used to test the effects of dietary exogenous antioxidants in vivo [17, 18]. The study of antioxidant is often associated with high stress resistance. A superoxide-generating compound, paraquat, and the general oxidative stressor H2O2 are frequently used [19-21]. In addition, a promising path to resolve H2O2 production in vivo is the use of the enzymes SOD and CAT [22, 23].
The aim of this article is to provide a detailed description of how to determine antioxidant activity by chemical probe both in vivo and in vitro for which the method has never been applied before. We illustrated this by demonstrating how the protocols established for the determination of antioxidants of lees. In the past few decades, the antioxidant activities of red wine have been widely reported [24-26]. However, wine lees, which is the residue formed at the bottom of containers of wine after fermentation, has not been studied adequately. In this study, a post-column-derived HPLC-UV-FLD approach was carried out to screen the antioxidant ingredients of lees that can rapidly react with H2O2 in vitro. Additionally, a robotic manipulation system combined with an NBCD fluorescent probe was used to determine the H2O2 levels in living Drosophila. The method provides a reference for the study of antioxidants in natural products and for use in studies of oxidative stress.
Lees was kindly provided by JF-Pharmaceutical Technology Development Co., Ltd. (Tianjin, China). The fluorescent probe NBCD N-borylbenzyloxycarbonyl-3, 7-dihydroxyphenoxazine) was kindly provided by Professor Zheng Yin, Nankai University (Tianjin, China). The Superoxide Dismutase Assay Kit was purchased from Jiancheng Bioengineering Institute (Nanjing, China). Catalase (CAT) assay kits, (4-hydroxyphenylacetic acid) PHPAA and paraquat were purchased from Sigma-Aldrich Co., (MO, USA). The primary antibodies anti-SOD1, anti-SOD2 and anti-CAT were obtained from Abcam (Cambridge, UK), and anti-β-actin was obtained from Cell Signaling Technology (Beverly, MA, USA). Goat anti-rabbit lgG (HRP) secondary antibodies were obtained from Abcam (Cambridge, UK). Caffeic acid, epicatechin, tartaric acid, gallic acid and quercetin were obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Chemiluminescent HRP substrates were purchased from Millipore Corporation (MA, USA). Liquid chromatography (LC)-grade formic acid was obtained from Acros Organics (Geel, Antwerp, Belgium). LC-grade acetonitrile was obtained from Fisher (Pittsburgh, PA, USA). LC water was purified using a MilliQ system (Millipore Corp, Bedford, MA, USA). The purity of all other reagents was analytical grade.
All assays were performed using ISO4 strain flies obtained from the laboratory of Professor Shian Wu, Nankai University (Tianjin, China). Flies were reared at 25 ℃ and 65% humidity on 12-hour light and dark cycles at a density of 20–30 flies per vial and transferred to new food every 2 to 3 days. The standard food used in all feeding assays was composed of 4.9% corn meal, 2.45% yeast, 6.72% glucose, 0.55% agar, and 0.3% nipagin. Three-day-old flies were collected using CO2, sorted by sex and transferred to vials on the designed diet for testing. For all experiments, at least 200 flies were used in each group, and each experiment was repeated two to four times.
To evaluate the effects of lees on fly sensitivity to oxidative stress, H2O2 and paraquat were used separately to induce high stress resistance. The flies were fed 0, 1.0, 2.5 and 5.0 mg/mL lees or 0.5 mmol/L tartaric acid, epicatechin and caffeic acid. Fourteen days later, fruit flies were starved for 2 h, and then flies were transferred to new vials that contained filter paper saturated with 200 mL of 20 mmol/L paraquat or 30% H2O2 diluted in a 6% glucose solution. The number of dead flies was counted every hour until all flies were sacrificed. Each stress model and sex group were analysed separately.
To prove the antioxidant activity of lees, three-day-old female flies were fed 0, 1.0, 2.5 and 5.0 mg/mL lees for 40 days, and the flies were selected randomly at day 0, 10, 20, 30, 40, and used to measure enzyme activities and expressions. Three-day-old female flies were fed 0, 0.1, 0.5 and 1.0 mmol/L of tartaric acid, epicatechin and caffeic acid for 14 days and used to detect enzyme activities. The fly samples were frozen in liquid N2 and stored at -80 ℃ until testing. Frozen samples were homogenized (10% w/v) with cold saline and then centrifuged at 12, 000 rpm for 15 min at 4 ℃. The supernatant was moved into a new tube for BCA protein assay (Solarbio, USA) and enzyme activity determination. The SOD and CAT activities were measured using an assay kit according to the manufacturer's protocol. The absorbance at 450 nm or 520 nm was recorded on a SpectraMax i3x Multi-Mode Detection Platform (MD, Austria).
Western blot analysis was conducted to investigate antioxidant enzyme expression. Frozen samples were homogenized (10% w/v) in ice-cold RIPA lysis buffer (Thermo, USA) and phenylmethanesulfonyl fluoride. The protein concentration was determined using a BCA protein assay kit. A 10% SDS–PAGE gel was transferred onto a PVDF membrane (Merck Millipore, Germany). The membrane was incubated in 5% skim milk for 2 h at 25 ℃ and then incubated with the primary antibody at 4 ℃ overnight. After washing the membrane with 1 × TBST 3 times, the membrane was incubated with a secondary antibody for 1 h at 37 ℃. After adequately washing the membrane with 1 × TBST, the membrane was tested with chemiluminescent HRP substrates (Santa Cruz Biotechnology, USA) and measured using a FluorChem E imager (ProteinSimple, Santa Clara, CA). The computer software Quantity One (Bio-Rad, CA, USA) was used to quantify densitometry. Antibodies used in this study were as follows: anti-SOD1 (1:3000), anti-SOD2 (1:2000), anti-catalase (1:3000), anti-β-actin (1:1000), and goat anti-rabbit lgG (HRP) (1:3000).
A post-column-derived HPLC-UV-FLD system was established to detect the reactive oxygen radical scavenging activity based on PHPAA chemiluminescence. The protocol was performed as previously described with slight modifications for antioxidant screening of the lees extract [27]. PHPAA can react with H2O2 and be converted to 2, 2'-dihydroxydiphenyl-5, 5'-iminodiacetic acid, which has fluorescent characteristics. HPLC-separated compounds that can scavenge H2O2 in lees reduced the fluorescent reaction product. All analyses were carried out on a LC-20CE Shimadzu liquid chromatograph system equipped with a SPD-20 A UV–vis detector and an RF-20 A FL–vis detector, and the measurements were conducted with two LC-20 AT solvent delivery pumps and two LC-10 AT derivatization solvent delivery pumps. Separation was achieved on a water symmetry C18 column (250 mm × 4.6 mm, i.d., 5 mm). The flow rate was set at 0.5 mL/min, and the mobile phase was a gradient of A (CH3CN) and B (1 × 10-3 mol/L H3PO4): 2%–13% A for 0-15 min, 13%-21% A for 15-25 min, 21%-25% A from 25 min to 30 min, 25%-31% A for 30-40 min, 31%-37% A for 40-50 min, 37%-43% A for 50-58 min, 43%-49% A for 58-65 min, 49%-100% A for 65-68 min and 100% A from 68 min to 70 min. The column temperature was maintained at 35 ℃ with the help of a column oven (CTO-10 AS). Ten milligrams of lees was ultrasonically extracted with 1 mL of 75% methanol for 30 min, and then the extract solutions were filtered through 0.42 mm filters. The injection volume of the test sample was 20 mL. The separated compounds were first detected at 260 nm using a UV detector and then directly entered into the subsequent derivation process. The post-column derivatization was based on a simultaneous supply of a 3% H2O2 solution and derivatization reagent that contained 8 × 10-6 mol/L hemin and 8×10-5 mol/L PHPAA dissolved in an NH4Cl/NH3H2O buffer solution (pH 10.5), and the flow rates were both set at 0.2 mL/min. The derivatization reaction was maintained in the length of a PEEK tube (0.18 mm × 3 m) at 25 ℃. The derived solution was then detected by a fluorescence detector. The excitation wavelength was 315 nm, and the emission wavelength was 400 nm. And the compounds in lees were detected and analysed using the UPLC-Q/TOF-MS method. The method is described in the Supporting Information. Then, HPLC analysis was used to identify the active ingredient by comparing its retention time with the standards. The concentration of the reference standard was 1.0 mg/mL, and the injection volume was 20 mL A robotic manipulation system was used to determine the H2O2 levels in living Drosophila. The system consisted of standard inverted fluorescence microscope (Nikon, Eclipse TI-E, Japan), a CCD mono camera (Balser, acA645-100 gm, Germany) for visual detection, an in-house developed pneumatic micro-injector to supply the injection pressure, a motor controller (Zolix, MC600-4B, China) to drive the motors of the micro-injector, a pair of 3-DOF motorized micromanipulators (Sutter, MP-285, USA) for positioning the micropipettes and a host computer for microscopic image processing and motion control. The CCD camera had a resolution of 640 × 480 pixels and a frame rate of 20 Hz. The micro-injector had a resolution of 2.5 Pa and a maximum output pressure of 150 kPa. The micromanipulators had a travel range of 25 × 25 × 25 mm, repeatability of 0.2 mm in low and 0.4 mm in high, as well as a maximum speed of 2.9 mm/s. The accurate inner diameter of the micropipette was measured, and the moving distance of the gasliquid interface (GLI) was calculated according to the injection volume [28].
The same H2O2 stress test described in H2O2 assay was repeated, and the exposure was performed in female flies with 30% H2O2 diluted in a 6% glucose solution for 6 h. After anaesthetizing flies with ether for 1 min, they were placed on a glass petri dish. A 5 mmol/L NBCD probe solution dissolved in dimethylsulphoxide (DMSO) was injected into the thorax using a micropipette with an inner diameter of approximately 20 mm, and the injection volume was 30 nL. The motor of the micro-injector was used to change the injection pressure until the GLI moved to the desired position. The procedure was described in a previous report [15]. The detection wavelength was achieved in the range of 190–400 nm. In the same manner, antioxidant active ingredients in lees were fed to flies to test H2O2 scavenging in living Drosophila. True fluorescent images of the flies were taken after 5 min and then converted to grey-scale images, and the degree of fluorescence intensity, which was difficult to distinguish visually, was determined by the Image Pro Plus software system (Mathworks, 2013b, USA). Then, H2O2 imaging was expressed based on the overall average grey value. Five images from each group were chosen to calculate the degree of staining.
The values shown represent the means ± standard deviation (SD). Data were analysed with GraphPad 6 using one-way ANOVA. Lifespan data were analysed using the log-rank test (Mantel-Cox Test). P < 0.05 was considered significant.
To explore the effect of lees on the vital force of flies, oxidative stress experiment was carried out. Paraquat specifically generates superoxide, whereas general oxidative stress is induced by H2O2. Therefore, flies were subjected to paraquat and H2O2. As shown in Figs. 1A and B, paraquat intensely reduced fly survival and killed approximately 50% of animals (females and males) in the control group after approximately 15 h of exposure. When flies were treated with lees, both female and male flies showed significantly better survival and mean survival during paraquat treatment, with the largest difference seen at a lees concentration of 2.5 mg/mL (Fig. 1C). As shown in Fig. 1D, 2.5 mg/mL lees-fed females displayed an approximately 30% higher survival rate than the control group after 15 h of H2O2 exposure. On the other hand, male flies did not show a significant difference in the H2O2-induced toxicity test over the dose range tested (Fig. 1E). Only female flies under H2O2 exposure showed a significantly better mean lifespan, and they reached the overall greatest improvement at a concentration of 2.5 mg/mL lees (Fig. 1F). Taken together, it seems that lees is excellent for converting superoxide to H2O2, but H2O2 is still harmful. Thus, the ultimate goal of resistance to oxidative damage may be H2O2 scavenging, and it is plausible to use a female fly model to investigate the H2O2-scavenging activity in subsequent evaluation experiments.

|
Download:
|
| Fig. 1. Antioxidant protection of lees against paraquat and hydrogen peroxide oxidative stress. Paraquat assay: (A) Females, P < 0.01, 1.0 mg/mL lees vs. Control; P < 0.001, 2.5 mg/mL lees vs. Control. (B) Males, P < 0.01, 1.0 mg/mL lees vs. Control; P < 0.001, 2.5 mg/mL lees vs. Control; P < 0.001, 5.0 mg/mL lees vs. Control. (C) Mean lifespan. Both female and male flies fed the 2.5 mg/mL lees-treated food exhibited a significant increase in survival after exposure to paraquat. Hydrogen peroxide assay: (D) Females, P < 0.05, 1.0 mg/mL lees vs. Control; P < 0.001, 2.5 mg/mL lees vs. Control. (E) Males. (F) Mean lifespan: Male flies on lees-treated food did not exhibit a significant increase in survival when exposed to hydrogen peroxide. *P < 0.05, **P < 0.01, and ***P < 0.001. Significant differences between the curves were analysed by log-rank test. Data are presented as the means ± SD, and differences compared to the control were considered significant at *P < 0.05, **P < 0.01 and ***P < 0.001 according to one-way ANOVA | |
Then, the activity of antioxidant enzymes SOD1, SOD2 and CAT were assessed to clarify the oxidation resistance of lees. For the treatment of 2.5 mg/mL lees diet, both SOD1 and SOD2 activities were significantly up-regulated at days 10 and 20 compared to the control group (Figs. 2A and B), while CAT activity was higher in the lees-treated group than in the control at day 30 (Fig. 2C). The present study also investigated the expression of SOD1, SOD2 and CAT in female flies at day 0, 10, 20, 30, 40. The expression level of SOD1 was higher in the lees-treated group than in the control at day 30 (Fig. 2D), but there was no significant difference in SOD2 expression levels between the control and the lees-treated group (Fig. 2E). CAT expression was higher in the lees-treated group than in the control at day 10 (Fig. 2F). The results indicated that lees can scavenge free radicals by increasing the activity of antioxidant enzymes.

|
Download:
|
| Fig. 2. Eff ects of less supplementation (2.5 mg/mL diet) on enzymatic activity and protein expression compared to the control diet. Enzyme activity: (A) SOD1. (B) SOD2. (C) CAT; Gene expression: (D) SOD1. (E) SOD2. (F) CAT. The band intensity was quantified by scanning densitometry and standardized with respect to β-actin. The data are shown as the mean ± SD and were analysed by one-way ANOVA. *P < 0.05, **P < 0.01 and ***P < 0.001 compared to the control group | |
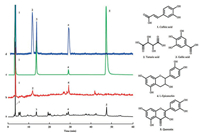
Having shown that lees increased antioxidant defence in Drosophila, it was necessary to determine which chemical components of lees were effective to protect Drosophila against oxidative damage. In this study, 18 chemicals were detected in lees by the UPLC-Q/TOF-MS method, including organic acids, hydrolysable tannins and flavonoids, and the results are described in Supporting information (Figs. S1, S2 and Table S1). The HPLC-UVFLD experiments involved separation and characterization of H2O2-scavenging compounds. The compounds in lees extract were separated by HPLC, and the peaks are shown in chromatogram a (Fig. 3). The compounds that had an inhibitory effect on the postcolumn fluorescence derivatization were recorded by a fluorescence detector and shown in chromatogram b, and three major peaks (peaks 1, 2 and 4) were chosen in the fluorescence spectra of lees to be investigated for chemical identification and antioxidant evaluation. The peaks 1, 2 and 4 were analysed as caffeic acid, tartaric acid and epicatechin by relative molecular mass and secondary mass spectra, and were identified by comparing the retention times with those of the standards in UV chromatography. Tartaric acid (peak 2) does not absorb at ultraviolet wavelengths in chromatogram a, but its activity in scavenging H2O2 can be evaluated in FLD chromatogram b. To explore the performance of the system, the peaks 3 and 5 in chromatogram a were identified as gallic acid and quercetin in the same way. The chromatogram c is a UV chromatogram of the five standards mixture, and their antioxidant activity was evaluated in FLD chromatogram d. The results showed that caffeic acid, tartaric acid and epicatechin of lees had a high H2O2-scavenging activity. Previous studies showed that the number of hydroxyl groups on the aromatic rings affects the antioxidant activity of phenolic acids [29, 30]. 1.0 mg/mL gallic acid was detected in the system, but gallic acid in lees had no effect, the concentration of gallic acid in lees was approximately 3 mg/mL, which is perhaps due to the influence of the detection limit of this method. Both quercetin in lees and 1.0 mg/mL quercetin did not show obvious H2O2-scavenging activity in the system, possibly because of the influence of reactivity. Overall, the HPLC-UV-FLD system detected chemicals in lees that can rapidly scavenge H2O2 in vitro, which may contribute to preventing in vivo H2O2-induced oxidative damage quicker and more effectively [31, 32].
|
Download:
|
| Fig. 3. HPLC-UV-FLD chromatogram of the sample extracted from lees and mixed standards of caffeic acid, tartaric acid, gallic acid, L-epicatechin and quercetin. a: HPLC-UV chromatogram of the sample extracted from lees; b: HPLC-FLD chromatogram of the sample extracted from lees. c: HPLC-UV chromatogram of mixed standards; d: HPLC-FLD chromatogram of mixed standards. Peaks and structures: 1, caffeic acid; 2, tartaric acid; 3, Gallic acid; 4, L-Epicatechin; 5, Quercetin | |
Furthermore, the kinetics of chemical and enzyme-catalysed reactions jointly scavenge H2O2 in the Drosophila body. To explore the effect of active ingredients of lees on antioxidant enzyme activity, the activity of SOD1, SOD2, and CAT were assessed. Previous studies have demonstrated that SOD dismutates superoxide radicals to form hydrogen peroxide, which inturn is decomposed to water and oxygen by CAT [33]. Regarding SOD1 activity (Fig. S2 A), 0.5 mmol/L and 1.0 mmol/L of tartaric acid and 0.1 mmol/L of epicatechin caused a significant upregulation in female flies compared to the control group. Additionally, 0.5 mmol/L and 1.0 mmol/L of tartaric acid and 0.1 mmol/L of epicatechin had a marked effect on SOD2 activity (Fig. S2B). Except for 0.5 mmol/L of tartaric acid, there is significant difference on CAT activity between the other treated group and the control group (Fig. S2C). Overall, the effects of tartaric acid, epicatechin and caffeic acid on SOD and CAT activities were different at different concentrations.
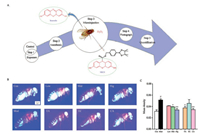
At last, to measure H2O2 levels in living organisms, it is important to clarify whether a chosen antioxidant actually reacts with a relevant oxidant. A previous study used the mitochondriatargeted ratiometric mass spectrometry probe MitoB to measure mitochondrial H2O2 production in living Drosophila and to determine the proportion of mitochondria in each fly body segment by separating whole flies into their component body segments [15]. In this study, NBCD was selective for H2O2 and did not react with other oxidants, and the injection process was not harmful. To derive more insight from the fluorescent images after the experimental procedures shown in Fig. 4A, we tested H2O2- scavenging activity in living Drosophila, and the results showed that there was a dose-dependent effect. Lees at 2.5 mg/mL and 5.0 mg/mL was sufficient to prevent female flies from damage under oxidative stress conditions. At concentrations of 0.5 mmol/L, tartaric acid and caffeic acid had greater effects on the H2O2 level in vivo than epicatechin (Figs. 4B and C). Epicatechin is known to be a biologically effective antioxidant in wine and tea and contributes to protecting the integrity of endothelial cells not only by scavenging free radicals but also by maintaining NO synthase [34, 35]. In this study, epicatechin supplementation had no significant effect on H2O2 levels in living flies, which suggests that the antioxidant activity of epicatechin mainly manifests in inhibition of NO synthesis rather than removal of HOO-. Combined, this outcome indicates that H2O2 in female flies might be modulated by the levels of tartaric acid and caffeic acid included in the experiment as the flies developed.
|
Download:
|
| Fig. 4. Outline of the strategy used to assess H2O2 within living flies using NBCD and fluorescent images. (A) The injection process, including the structure of NBCD and its product, Resorufin, which was formed by reaction with H2O2. (B) Fluorescent image 5 min after injection. (C) The average H2O2 level of flies fed diets containing 0 (Con), 1.0 mg/mL (Low), 2.5 mg/mL (Mid) and 5.0 mg/mL (Hig) lees and 0.5 mmol/L of tartaric acid (TA), epicatechin (EC) and caffeic acid (CA). Flies fed basic diets exposed to H2O2 were a model control (Mod). The data are presented as the means ± SD and were analysed by one-way ANOVA (n = 3). #P < 0.001 compared to the control group. *P < 0.05 and **P < 0.01 compared to the model group | |
In conclusion, the post-column-derived HPLC-UV-FLD system can screen nature antioxidants those rapidly scavenge H2O2 in vitro. Tartaric acid, epicatechin and caffeic acid of lees were revealed by the method. And the robotic manipulation system combined with an NBCD fluorescent probe can be used to show the level of H2O2 in living Drosophila. By using this, H2O2 can be specific selected without the effect of other oxides and peroxidase in vivo. And the "detection online" and "visualization" are realized through microscopic imaging. The result shown that the effect of lees on scavenging H2O2 in living Drosophila was associated with the components caffeic acid and tartaric acid. Taken together, both chemical and biological functions can be detected simultaneously based on the system. And the novel integrated system has great potential for screening and evaluating antioxidants from natural products contribute to the rapid scavenging of H2O2 in vitro and in vivo.
AcknowledgmentsThis work was financially supported by the Collaborative Innovation Center of Research on Functional Ingredients from Agricultural Residues (Guangxi University of Chinese medicine, No. CICAR 2015-B2). The authors appreciate the helpful advice and support from Associate Professor Luqing Shang (College of Pharmacy, Nankai University) on NBCD fluorescent probe.
Appendix A. Supplementary dataSupplementarymaterial related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.05.020.
| [1] |
T. Ishikawa, S. Tamaki, T. Maruta, S. Shigeoka, Adv. Exp. Med. Biol. 979 (2017) 47. DOI:10.1007/978-3-319-54910-1 |
| [2] |
M. Schieber, N.S. Chandel, Curr. Biol.:Cb 24 (2014) R453-R462. DOI:10.1016/j.cub.2014.03.034 |
| [3] |
X. Liao, Y.A. Liu, Curr. Org. Chem. 17 (2013) 654-669. DOI:10.2174/1385272811317060008 |
| [4] |
A.A. Karaçelik, M. Küçük, Z. İskefiyeli, et al., Food Chem. 175 (2015) 106-114. DOI:10.1016/j.foodchem.2014.11.085 |
| [5] |
J. Damašius, P.R. Venskutonis, V. Kaškoniene, A. Maru ška, Analyt. Methods 6 (2014) 2774-2779. DOI:10.1039/c3ay41703d |
| [6] |
J.D.V.D. Merwe, E. Joubert, M. Manley, et al., Food Chem. Toxicol. 50 (2012) 808-815. DOI:10.1016/j.fct.2011.11.018 |
| [7] |
G.G. Guilbault, Anal. Chem. 40 (1968) 459R-471R. |
| [8] |
B. Qi, Y.Z. Zhu, M. Hu, Y. Zhang, X. Tang, Anal. Lett. 34 (2001) 1247-1254. DOI:10.1081/AL-100104150 |
| [9] |
I. Navarrogonzález, R. Gonzálezbarrio, V. Garcíavalverde, et al., Int. J. Mol. Sci. 16 (2014) 805-822. DOI:10.3390/ijms16010805 |
| [10] |
K.A. Lukyanov, Nat. Methods 3 (2006) 281-286. DOI:10.1038/nmeth866 |
| [11] |
M. Gutscher, M.C. Sobotta, G.H. Wabnitz, et al., J. Biol. Chem. 284 (2009) 31532. DOI:10.1074/jbc.M109.059246 |
| [12] |
K.N. Markvicheva, D.S. Bilan, N.M. Mishina, et al., Biorg. Med. Chem. 19 (2011) 1079-1084. DOI:10.1016/j.bmc.2010.07.014 |
| [13] |
D.S. Bilan, L. Pase, L. Joosen, et al., ACS Chem. Biol. 8 (2013) 535-542. DOI:10.1021/cb300625g |
| [14] |
K. Liu, H. Shang, X. Kong, et al., Biomaterials 100 (2016) 162. DOI:10.1016/j.biomaterials.2016.05.029 |
| [15] |
H.M. Cochemé, A. Logan, T.A. Prime, et al., Nat. Protoc. 7 (2012) 946-958. DOI:10.1038/nprot.2012.035 |
| [16] |
Z. Han, X. Liang, X. Ren, L. Shang, Z. Yin, Chem-Asian J. 11 (2016) 818-822. DOI:10.1002/asia.v11.6 |
| [17] |
Y.X. Zou, M.H. Ruan, J. Luan, et al., J. Nutr. Health Aging 21 (2016) 1-6. |
| [18] |
De Aguiar L.M., F.H. Figueira, M.S. Gottschalk, R.C. Da, Comp. Biochem. Phys. C 185- 186 (2016) 94-101. |
| [19] |
P.B. Bagatini, L. Saur, M.F. Rodrigues, et al., Invert. Neurosci. 11 (2011) 43. DOI:10.1007/s10158-011-0116-3 |
| [20] |
X. Sun, T. Komatsu, J. Lim, et al., Aging Cell. 11 (2012) 783-793. DOI:10.1111/j.1474-9726.2012.00842.x |
| [21] |
C. Peng, H.Y. Chan, Y. Huang, H. Yu, Z.Y. Chen, J. Agric. Food Chem. 59 (2011) 2097-2106. DOI:10.1021/jf1046267 |
| [22] |
J. Dasgupta, S. Subbaram, K.M. Connor, et al., Antioxid. Redox Signal. 8 (2006) 1295-1305. DOI:10.1089/ars.2006.8.1295 |
| [23] |
G. Patro, S.K. Bhattamisra, B.K. Mohanty, H.B. Sahoo, Pharm. Res. 8 (2016) 22. |
| [24] |
M.V. Baroni, R.D.D.P. Naranjo, C. García-Ferreyra, S. Otaiza, D.A. Wunderlin, LWT Food Sci. Technol. 47 (2012) 1-7. DOI:10.1016/j.lwt.2012.01.015 |
| [25] |
E. Kurin, P. Mucaji, M. Nagy, Molecules 17 (2012) 143369-14348. |
| [26] |
Y. Niu, L. Yin, S. Luo, et al., Phytochem. Anal. 24 (2013) 59-68. DOI:10.1002/pca.v24.1 |
| [27] |
J. Hong, J. Maguhn, D. Freitag, A. Kettrup, FreseniusJ.Anal.Chem. 361 (1998) 124-128. DOI:10.1007/s002160050847 |
| [28] |
N. Li, Y. Liu, S. Li, et al., Mod. Phys. Lett. B 31 (2017) 1750148. |
| [29] |
Z. Sroka, W. Cisowski, Food Chem. Toxicol. 41 (2003) 753-758. DOI:10.1016/S0278-6915(02)00329-0 |
| [30] |
S.Z. Gorjanović, M.M. Novaković, N.I. Potkonjak, D.Z. Suznjević, J. Agric. Food Chem. 58 (2010) 4626. DOI:10.1021/jf100022e |
| [31] |
A.V. Peskin, A.G. Cox, P. Nagy, et al., Biochem. J. 432 (2010) 313-321. DOI:10.1042/BJ20101156 |
| [32] |
N.E. Gislason, B.L. Currie, A.L. Waterhouse, J. Agric. Food Chem. 59 (2011) 6221-6226. DOI:10.1021/jf200115y |
| [33] |
M. Wołonciej, E. Milewska, W. Roszkowska-Jakimiec, Postepy. Hig. Med. Dosw. 70 (2016) 1483. DOI:10.5604/17322693.1229074 |
| [34] |
M.M. Cals-Grierson, Patent, WO0182929, 2001.
|
| [35] |
Y. Steffen, T. Schewe, H. Sies, Biochem. Biophys. Res. Commun. 331 (2005) 1277-1283. DOI:10.1016/j.bbrc.2005.04.035 |
 2018, Vol. 29
2018, Vol. 29 


