b Liaoning Center of Disease Prevention and Control, Shenyang 110001, China
Sodium hypochlorite (NaClO), as well known as famous bactericide and oxidizing agent, play vital roles in many fields such as drinking water disinfection [1, 2], dye decoloration [3, 4], odor elimination [5], oxidation contaminates in soils [6, 7], and so on. Especially, in water treatment, e.g., reclaimed water and drinking water, sodium hypochlorite is now among the most widely used disinfectant, owing to its residual protection, low cost and ease of use [8-10]. The free chlorine residual can guarantee to kill bacteria and inactivate viruses, but it also can react with the constituents present to form disinfection by-products (DBPs) during water disinfection [11-13]. Some DBPs (e.g., N-nitrosamines, trihalomethanes, haloacetic acids, haloacetonitriles, etc.) are of growing concern because they have been recently identified as probable human carcinogens and deformity [14, 15]. Therefore, a reasonable control on free chlorine residual in water disinfection will be of great significance to guarantee for the health of populations. Besides that, hypochlorous acid produced by the enzyme myeloperoxidase in inflammatory stress [16] plays a critical role as one of the most important reactive oxygen species (ROS) in defending from the invasion of contaminants [17]. On the contrary, uncontrolled endogenous hypochlorite will further induces oxidation damage of the extracellular components, which may involve in some diseases, such as arthritis [18], kidney disease [19], lung injury [20], atherosclerosis [21] and cancer [22]. As well known, both hypochlorous acid and hypochlorite anion exist in vivo at almost equal concentrations. Measurement of hypochlorous acid and hypochlorite constitutes a fundamental aspect to evaluate the stress state exposed to different hazardous contaminants. Given the health risk of ClO-, it is necessary to test and monitor hypochlorite residues both in real water samples and in biological specimens.
Right now, colorimetric and fluorometric methods are available for detecting and monitoring hypochlorite residues in water [23, 24]. Colorimetric methods that utilize color changes to quantitatively assess residual hypochlorite against a visual comparator standards or by usage of a colorimeter make themselves very adept in facilitating hypochlorite detection in real water samples but not in vivo. Fluorometric methods can offset this imperfection in monitoring hypochlorite produced from intracellular micro-environment of flora and fauna. Developing colorimetric and fluorometric probes for hypochlorite is still highly demanded yet challenging, though a few probes for specific detection of hypochlorite have been reported [25-29]. Recently, rhodamine dyes have been the preferred chromophore and fluorophore in the design of colorimetric and fluorometric probes, owing to its brilliant red color, high quantum yields and excellent photophysical properties [30-33]. However, most of the reported rhodamine-based probes for ClO- displayed either modest selectivity or delayed response time. Thus, it is still urgently needed to develop rapidly responsive, highly selective and sensitive fluorescent probes for ClO-, which can be used in both colorimetric and fluorometric tests.
Herein, a rapidly responsive, selective and sensitive fluorescent probe for hypochlorite based on rhodamine B fluorophore has been developed and utilized to detect hypochlorite in real sample and living cells. A two-step-in-one-pot synthesis of Rh-ClO was very concise and highly efficient. Reaction of rhodamine B with POCl3 followed by thiophene-2-carbohydrazide afforded Rh-ClO with 62% yield (Scheme 1). It is reported that hydrazide can be oxidized by hypochlorite and hydrolyze into carboxylic acid in aqueous solution [34].Recently, rhodaminedyes have been widely employed to design 'turn-on' fluorescent probes for various analytes [35-40], owing to their outstanding ring-opening transformations from colorless and non-fluorescent spirolactam forms to rhodamine fluorophores with intensive absorptions and emissions in the visible spectrum range [30, 31]. Thereupon, thiophene-2-carbohydrazide has been introduced into rhodamine B fluorophore through the spirolactam connection pattern to develop color and fluorometric probe Rh-ClO for hypochlorite.
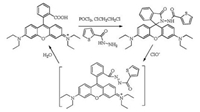
|
Download:
|
| Scheme 1. Synthetic route of Rh-ClO | |
Anion selectivity studies were first performed in Tris-HCl buffer solution (C2H5OH/H2O = 4/6 (v/v), pH 7.4). The presence of 100 equiv. anions and reactive species such as ONOO-, Cl-, HPO42-, H2PO4-, SO42-, NO3-, CO32-, S2-, S2O32-, H2O2, NO and ·OH, did not cause any color changes of solution, which meant that the ringopening reaction of spirolactam was not brought about in presence of these species, as show in Figs. 1a and b. Upon addition of 100 equiv. ClO-, the solution color changed from colorless to pink and was immediately visible to the naked-eyes, and a red fluorescence was observed under excited at UV 365 nm light. Meanwhile, a new absorption peak at 556 nm and fluorescent peak at 578 nm appeared, which indicated that hypochlorite could induce a ring-opening reaction of rhodamine moiety, and the product rhodamine B had already generated, which was verified by HRMS (Fig. S1 in Supporting information). Furthermore, in Tris-HCl buffer solution (C2H5OH/buffer = 4/6 (v/v), pH 7.4), the presence of 100 equiv. various metal ions, such as K+, Ca2+, Na+, Mg2+, Fe2+, Fe3+, Cu2+, Zn2+, Al3+, Cd2+, did not cause any observable changes of absorption and emission spectra (Fig. S2 in Supporting information). The effect of competitive anions was also studied by adding hypochlorite to Rh-ClO solution in the presence of anions and reactive species, such as ONOO-, Cl-, HPO42-, H2PO4-, SO42-, NO3-, CO32-, S2-, S2O32-, H2O2, NO, ·OH, which were prepared and calibrated according to the methods reported in supplementary. The presence of anions and reactive species did not affect absorption and emission spectral responses between Rh-ClO and ClO- (Fig. S3 in Supporting information). The good selectivity and anti-interference for ClO- indicated that Rh-ClO would be a potential tool as both colorimetric and fluorometric probe for ClO- test in practice.

|
Download:
|
| Fig. 1. Absorption (a) and emission (b) spectra of Rh-ClO in the presence of different anions. Changes in the absorption (c) and emission spectra (d) of Rh-ClO (20 mmol/L) in C2H5OH/H2O (4/6, v/v) at pH 7.4 (0.1 mmol/L Tris-HCl buffer) with increasing of NaOCl solution from 0 to 300 mmol/L. (c1, d1) The response of absorption intensity (556 nm) and emission intensity (578 nm) in the concentration of NaOCl from 0 to 300 mmol/L | |
In order to further assess pH effect, the absorption and emission spectral changes of Rh-ClO for pH were studied (Fig. S4 in Supporting information). When the pH value was above 6.0, there was no absorption peak in C2H5OH-H2O (4/6, v/v) solution of RhClO, indicating that Rh-ClO was suitable for usage at neutral and basic conditions. With the decrease of pH value, a new absorption peak at 556 nm was gradually enhanced, indicating that spirolactam of Rh-ClO underwent a ring-opening reaction in the protonation of Rh-ClO. Meanwhile, Rh-ClO showed 300-folds enhancement of fluorescent intensity with attenuation of pH values from 6.0 to 3.5. The pKa value of probe Rh-ClO was 4.76 (± 0.02), calculated from fluorescence titration data. Upon addition of 100 equiv. ClO- into Rh-ClO solution for 3 min, there were not clear changes in absorption spectra of Rh-ClO over a wide range of pH value from 3.5 to 13.0. By contrast, the emission spectra of Rh-ClO remained unchanged in pH range from 6.0 to 12.5. When the pH further decreased (3.5–6.0), the emission intensity at 578 nm reduced slowly, which was consistent with that performance of rhodamine B in the same pH range.
Hypochlorite titration experiments were carried out in the solution of ethanol and Tris-HCl buffer (C2H5OH/H2O = 4/6 (v/v), pH 7.4). With increasing of NaClO concentration, the solution of Rh-ClO exhibited intensive magenta color and a new absorption peak at 556 nm increases gradually (Fig. 1c). Meanwhile, the emission peak at 578 nm gradually enhanced (Fig. 1d). The absorption intensity at 556 nm was linear function with NaClO concentration over the range of 0–120 mmol/L (Fig. c1). By contrast, in the range of NaClO concentration from 0 to 40 mmol/L, there was a linear enhancement of emission intensity at 578 nm (Fig. d1). The response mechanism involved the oxidation reaction between the thiophene-2-carbohydrazide moiety of Rh-ClO and hypochlorite at neutral pH condition (Scheme 1). ClO- could oxidate acylhydrazine group and induce a ring-opening process in rhodamine moiety of Rh-ClO. Then hydrolyzed product rhodamine B was subjected to ESI-MS analysis in which the peak at m/z 443.2329 was found (Fig. S1).
The detection limit (DL) was calculated from the absorption and fluorescence titration data (Fig. S5 in Supporting information). Over the ClO- range from 40 mmol/L to 220 mmol/L, a good linear regression curve between hypochlorite concentration and the function of normalized maximum and minimum absorption was fitted (R = 0.993), and the point at which this line crossed the ordinate axis was taken as the detection limit and equal approximately 3 ×10-5 mol/L (DLabs). According to fluorescent titration of Rh-ClO, there was also a good linear regression curve in the range of ClO- from 9 mmol/L to 90 mmol/L (R = 0.991). The detection limit of Rh-ClO was determined to be 7 × 10-6 mol/L (DLfl). Compared with DLabs, the detection limit of fluorescence titration (DLfl) was much lower, which indicated that fluorescent detection would be more highly sensitive. The reaction time between Rh-ClO and ClO- was investigated in spectrofluorophotometer (Fig. S6 in Supporting information). Time-dependent intensity at 578 nm of Rh-ClO was recorded in absence and presence of ClO-. In the absence of NaClO, Rh-ClO showed a negligible background fluorescence that was stable under the assay conditions. Upon addition of 30 mmol/L NaClO, the intensity of Rh-ClO at 578 nm achieved the maximum within 5 s, and then decreased slightly. The fluorescent intensity leveled off after 8 s of adding NaClO. With further addition of NaClO (0.05 mmol/L, 0.1 mmol/L, 0.15 mmol/L, respectively), the intensity of Rh-ClO leveled off within 8 s. Rh-ClO exhibited a fast response with NaClO, which should be ascribed to the electron-withdrawing characteristic of thiophene-2-carbohydrazide moiety in Rh-ClO. The electron-withdrawing characteristic of substituent could lead to an accelerating effect on the HOCl-mediated oxidation-hydrolysis of hydrazide in aqueous solution [41].
In order to investigate the practical application of Rh-ClO for the detection of hypochlorite anion, aqueous solutions of Rh-ClO (2.0 × 10-5 mol/L) and filter paper strips test were performed. RhClO in ethanol was added into vials of different anions in aqueous solution, respectively. When the color of vial changed from colorless to pink, it indicated that there was hypochlorite anion in the second vial (Fig. 2a). To ease usage of probe Rh-ClO, test-paper has been prepared and used to detection ClO- in real sample. After test strips were immersed in ClO- solution with various concentrations for 10 s, respectively, it could be found there was an obvious color deepening with the increasing concentration of hypochlorite solutions (0, 0.02, 0.06, 0.2 mol/L) (Fig. 2b). These results indicated that Rh-ClO could be a handy tool to be used as a colorimetric probe in the fast detection of hypochlorite in aqueous solution.

|
Download:
|
| Fig. 2. (a) Color detection of absorption and emission on hypochlorite among different anions in aqueous solution (0.05 mol/L, C2H5OH/H2O (4/6, v/v)). (b) Test papers soaked with Rh-ClO was immersed in 0, 0.02, 0.06 and 0.2 mol/L NaClO in distilled water, respectively | |
As above mentioned, hypochlorite anion produced in inflammatory stress plays a vital role in defending from the invasion of pathogens. To further examine the possibility of application in living cells, fluorescent images of HeLa cells stained with Rh-ClO were obtained by a laser-scanning confocal microscopy. Upon excitation at 559 nm, dim red intracellular fluorescence was displayed in red channel (red channel BF: 565–665 nm) (Fig. 3a). Stimulated by addition of sodium hypochlorite (50 mmol/L) for 5 min, stronger fluorescence was observed and distributed in subcellular locations of cells (Fig. 3b). In order to further validate organelle-targeting of Rh-ClO in HeLa cells, co-localization experiments were studied by HeLa cells co-stained with Rh-ClO and Rh 123, a mitochondrial tracker. After stained with 5 mmol/L Rh-ClO and 2 mmol/L Rh123, HeLa cells respectively illustrated green and red fluorescence in channels 1 and 2 (Fig. 3c, ch1 and ch2). The well merged image of ch2 and ch1 suggested that Rh-ClO could specifically accumulate in mitochondria of HeLa cells. The Pearson's coefficient and Mander's overlap coefficient were up to 0.82 and 0.91, respectively. Moreover, a high correlation-ship demonstrated in intensity scatter plot of ch1 and ch2 indicated RhClO existed predominantly in mitochondria of HeLa cells (Fig. 3d). Selected a red rectangular section on intensity scatter plot allowed us to highlight corresponding white pixels on simulate images in accordance with the overlay image (Fig. 3e). These results demonstrated that Rh-ClO could potentially be used as a nondestructive indicator for hypochlorite monitoring in toxicological experiments.

|
Download:
|
| Fig. 3. Fluorescent image of HeLa cells stained with Rh-ClO (5.0 mmol/L) in absence (a) and presence (b) of sodium hypochlorite. (c) Rh-ClO co-localizes to mitochondria in HeLa cells. HeLa was stained with Rh 123 (2.0 mmol/L, Channel 1 ch1): λex 488 nm, λem 500–540 nm) and Rh-ClO (5.0 mmol/L, Channel 2 ch2): λex 559 nm, λem 565–665 nm) with sodium hypochlorite solution (50 mmol/L) for 5 min at 37 ℃. Merged is overlay image of ch1 and ch2. DIC is differentia interference contrast image. (d) Intensity scatter plot of ch1 and ch2. (e) Simulate image, the white pixels represent by the scatter plot is highlighted by selecting the points with a red rectangular selection | |
In summary, Rh-ClO, as a colorimetric and fluoromeric probe, has been easily obtained from the reaction of rhodamine B with thiophene-2-carbohydrazide. Probe Rh-ClO displayed a high selectivity and sensitivity to hypochlorite anion in aqueous solution. The detection limit of Rh-ClO was 7 μmol/L, fitted by fluorescent titration. Response time of Rh-ClO with hypochlorite anion was as short as ten seconds. Moreover, probe Rh-ClO and paper test were successfully used to determine ClO- in real water samples. Finally, co-localization experiments of HeLa cells further confirmed that Rh-ClO could detect exogenous ClO- in mitochondria of living cells. These results suggested that the new probe would lead to the opportunities not only for determining ClO- in water and biological samples, but also for the evaluation of oxidation stress induced by contaminants.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21302080) and Program Funded by Liaoning Province Education Administration (No. L2014010).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.01.054.
| [1] |
P.K. Roy, D. Kumar, M. Ghosh, A. Majumder, Desalin. Water Treat. 57 (2016) 28141-28150. DOI:10.1080/19443994.2016.1183522 |
| [2] |
J.Q. Jiang, B. Lloyd, Water Res. 36 (2002) 1397-1408. DOI:10.1016/S0043-1354(01)00358-X |
| [3] |
T. Omura, Dyes Pigm. 26 (1994) 33-50. DOI:10.1016/0143-7208(94)80028-6 |
| [4] |
R.X. Yuan, S.N. Ramjaun, Z.H. Wang, J.S. Liu, J. Hazard. Mater. 196 (2011) 173-179. DOI:10.1016/j.jhazmat.2011.09.007 |
| [5] |
S.T. Chang, M.S. Chou, H.Y. Chang, Aerosol. Air Qual. Res. 14 (2014) 293-300. DOI:10.4209/aaqr.2013.01.0014 |
| [6] |
A.J. Renneberg, M.J. Dudas, Waste Manage. Res. 20 (2002) 468-475. DOI:10.1177/0734242X0202000510 |
| [7] |
F. Picard, J. Chaouki, Chemosphere 145 (2016) 200-206. DOI:10.1016/j.chemosphere.2015.11.040 |
| [8] |
W.A. Rutala, D.J. Weber, Clin. Microbiol. Rev. 10 (1997) 597-610. DOI:10.1128/CMR.10.4.597 |
| [9] |
J. Koivunen, H. Heinonen-Tanski, Water Res. 39 (2005) 1519-1526. DOI:10.1016/j.watres.2005.01.021 |
| [10] |
Y.G. Feng, D.W. Smith, J.R. Bolton, J. Environ. Eng. Sci. 6 (2007) 277-284. DOI:10.1139/s06-052 |
| [11] |
J.Y. Fang, X. Yang, J. Ma, C. Shang, Q.A. Zhao, Water Res. 44 (2010) 5897-5906. DOI:10.1016/j.watres.2010.07.009 |
| [12] |
S. Monarca, S.D. Richardson, D. Feretti, et al., Environ. Toxicol. Chem. 21 (2002) 309-318. DOI:10.1002/etc.v21:2 |
| [13] |
I.M. Schreiber, W.A. Mitch, Environ. Sci. Technol. 41 (2007) 7039-7046. DOI:10.1021/es070500t |
| [14] |
S.D. Richardson, M.J. Plewa, E.D. Wagner, R. Schoeny, D.M. DeMarini, Mutat. Res. Rev. Mutat. 636 (2007) 178-242. DOI:10.1016/j.mrrev.2007.09.001 |
| [15] |
R. Crebelli, L. Conti, S. Monarca, et al., Water Res. 39 (2005) 1105-1113. DOI:10.1016/j.watres.2004.12.029 |
| [16] |
A. Hammer, G. Desoye, G. Dohr, W.G. Sattler, E. Malle, Lab Invest. 81 (2001) 543-554. DOI:10.1038/labinvest.3780263 |
| [17] |
Z.M. Prokopowicz, F. Arce, R. Biedron, et al., J. Immunol. 184 (2010) 824-835. DOI:10.4049/jimmunol.0902606 |
| [18] |
S.M. Wu, S.V. Pizzo, Arch. Biochem. Biophys. 391 (2001) 119-126. DOI:10.1006/abbi.2001.2408 |
| [19] |
E. Malle, T. Buch, H.J. Grone, Kidney Int. 64 (2003) 1956-1967. DOI:10.1046/j.1523-1755.2003.00336.x |
| [20] |
S. Hammerschmidt, N. Buchler, H. Wahn, Chest 121 (2002) 573-581. DOI:10.1378/chest.121.2.573 |
| [21] |
C. Bergt, S. Pennathur, X.Y. Fu, et al., Proc. Natl. Acad. Sci.U.S.A. 101 (2004) 13032-13037. DOI:10.1073/pnas.0405292101 |
| [22] | |
| [23] |
A. Murray, D. Lantagne, J. Water Health 13 (2015) 79-90. DOI:10.2166/wh.2014.195 |
| [24] |
J. Zhang, X.L. Wang, X.R. Yang, Analyst 137 (2012) 2806-2812. DOI:10.1039/c2an35239g |
| [25] |
X.Q. Chen, F. Wang, J.Y. Hyun, et al., Chem. Soc. Rev. 45 (2016) 2976-3016. DOI:10.1039/C6CS00192K |
| [26] |
K. Setsukinai, Y. Urano, K. Kakinuma, H.J. Majima, T. Nagano, J. Biol. Chem. 278 (2003) 3170-3175. DOI:10.1074/jbc.M209264200 |
| [27] |
W.Y. Lin, L.L. Long, B.B. Chen, W. Tan, J. Chem. Eur. 15 (2009) 2305-2309. DOI:10.1002/chem.v15:10 |
| [28] |
S.T. Manjare, J. Kim, Y. Lee, D.G. Churchill, Org. Lett. 16 (2014) 520-523. DOI:10.1021/ol403405n |
| [29] |
J. Lv, Y. Chen, F. Wang, et al., Dyes Pigm. 148 (2018) 353-358. DOI:10.1016/j.dyepig.2017.09.037 |
| [30] |
S.S. Ding, Q. Zhang, S.H. Xue, G.Q. Feng, Analyst 140 (2015) 4687-4693. DOI:10.1039/C5AN00465A |
| [31] |
H.N. Kim, M.H. Lee, H.J. Kim, J.S. Kim, J. Yoon, Chem. Soc. Rev. 37 (2008) 1465-1472. DOI:10.1039/b802497a |
| [32] |
T. Zheng, X. Ding, Y. Liu, Z. Zhao, B. Zhao, RSC Adv. 5 (2015) 99664-99668. DOI:10.1039/C5RA20188H |
| [33] |
H. Yu, G. Li, B. Zhang, et al., Dyes Pigm. 133 (2016) 93-99. DOI:10.1016/j.dyepig.2016.05.028 |
| [34] |
M. Beija, C.A.M. Afonso, J.M.G. Martinho, Chem. Soc. Rev. 38 (2009) 2410-2433. DOI:10.1039/b901612k |
| [35] |
J.T. Hou, M.Y. Wu, K. Li, et al., Chem. Commun. 50 (2014) 8640-8643. DOI:10.1039/C4CC02673J |
| [36] |
Y. Yue, F. Huo, C. Yin, J.O. Escobedo, R.M. Strongin, Analyst 141 (2016) 1859-1873. DOI:10.1039/C6AN00158K |
| [37] |
Y. Chen, T. Wei, Z. Zhang, et al., Chin. Chem. Lett. 28 (2017) 1957-1960. DOI:10.1016/j.cclet.2017.05.010 |
| [38] |
N. Jiang, J.L. Fan, F. Xu, et al., Angew. Chem. Int. Ed. 54 (2015) 2510-2514. DOI:10.1002/anie.201410645 |
| [39] |
K. Li, J.T. Hou, J. Yang, X.Q. Yu, Chem. Commun. 53 (2015) 5539-5541. |
| [40] |
S. Ding, Q. Zhang, S. Xue, G. Feng, Analyst 140 (2015) 4687-4693. DOI:10.1039/C5AN00465A |
| [41] |
Z. Zhang, Y. Zou, C. Deng, L. Meng, Luminescence 31 (2015) 997-1004. |
 2018, Vol. 29
2018, Vol. 29 


