pH, potential of hydrogen, is an important indicator of organisms. Specific pH values are vital in many physiological and pathological processes [1, 2]. pH fluctuations may deeply disrupt renal and respiratory function and are closely related to various human diseases including neurodegenerative disorders, cardiovascular diseases and cancers [3, 4].
Precisely and dynamically monitoring pH values of biosamples could provide useful information for understanding cellular functions, biological research and the diagnosis and treatment of diseases [5, 6]. Compared to the traditional methods such as pH test paper [7], glass electrode [8], fluorescent probes have attracted much interest in view of their ease of operation, high sensitivity, real time and visualization, especially bioimaging in living cells [9-11]. However, small weighted organic fluorescent probes suffered from the defects of low solubility, poor cell permeability, ease of escape from cells [12, 13]. It is of particular significance to develop fluorescent probes for proton with excellent performance. Fluorescent nano-probes synthesized by covalently connecting organic probes to the surface of SiO2 nano-particles (NPs) have been applied in the areas of cytological markers, physiological test, biosensing and medical tracking due to the following advantages [14-18]: (1) Self-calibration could be realized by introducing fluorescent probe and reference dye molecules to the NPs simultaneously; (2) the quantum yield increases with the amount of probe molecules; (3) high stability and difficult leakage from biological samples. However, there are few reports on fluorescent SiO2 nano-probes, which attracted our attention to this research area.
Naphthalimide is a common used fluorophore in designing probes for various analytes because of its ease of modification and stable photophysical properties [19-24]. Herein, two small weighted probes A and B bearing terminal alkynyl with naphthalimide as the fluorophore and N, N-dimethylethylenediamine as the receptor were synthesized. Six kinds of fluorescent nano-probes for pH were prepared by bonding A and B to the surface of SiO2 NPs with different particle sizes through click reaction. The results show that particle size and the linkage play important roles in the performance of the nano-probes.
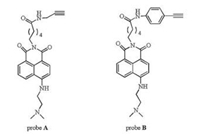
Small weighted probes A and B (Scheme 1), with 1.8- naphthalimide as the fluorophore and N, N-dimethylethylenediamine as the receptor for proton, were designed based on the photo-induced electron transfer (PET) mechanism. The synthesis procedures are illustrated in Supporting information. NMR and MS of A and B are shown in Figs. S1 and S2 (Supporting information). The tertiary amine N atom substituted with two methyl groups is a strong electron donor. Before protonation, strong photo-induced electron transfer takes place from the electron-rich dimethylsubstituted N to the fluorophore, consequently, the fluorescence is quenched. After protonation, the PET process is restrained; as a result, the fluorescence will be recovered. In addition, the protonation of the tertiary amine N lowers the electron pushpull effect of the probe leading to a slight blue-shift in both absorption and emission maxima. The substitutes at imide N in probes A and B are different, that in probe B is longer and more rigid. When the probes A and B covalently attached to the nanoparticles, the particle size, length and flexibility of the linker could affect the photophysical properties of the probe. In this work, the probes A and B were linked to nano-particles with different sizes to investigate the effects of particle size, the length and rigidity of the linker on the performance of the nano-probes.
|
Download:
|
| Scheme 1. The chemical structures of probes A and B. | |
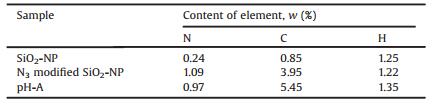
The SiO2 NPs prepared by in-situ generation were characterized by TEM and elemental analysis. Table 1 is the elemental analysis results of SiO2 nano-particles (SiO2-NPs), azide modified SiO2-NP and the fluorescent nano-probe (pH-A). It is clear that the contents of C and N in N3 modified SiO2-NP were much higher than those in SiO2-NP. The introduction of probe A made N content decrease slightly (from 1.09% to 0.97%) and the C content increase further (from 3.95% to 5.45%). The above results proved that the N3 and the fluorescent probe A were covalent modified to the SiO2 NPs successfully.
|
|
Table 1 The elemental analysis of three kinds of nano-particles |
The TEM images (Fig. 1) demonstrated that the SiO2 NPs were regularly spherical in the shape, homogeneous distribution with average sizes of 120 nm (left) and 300 nm (right), respectively. Six kinds of fluorescent nano-probes were obtained by covalently connecting probe A or B to azide modified SiO2 NPs with sizes of 20 nm, 120 nm and 300 nm (illustrated in Scheme S2) through click reaction and were characterized by fluorescence and UV–vis spectroscopy. The spectral properties of the fluorescent nanoparticles were similar to those of the probes A and B, revealing that A and B were successfully connected to the SiO2 NPs.

|
Download:
|
| Fig. 1. TEM images of two kinds of SiO2 NPs. | |
The absorption spectra of probes A and B in solutions with different pH were studied and the results are shown in Fig. 2. In acidic and neutral media, the absorption (Fig. 2a) and emission (Fig. 2b) maxima of the probe A are at 434 nm and 529 nm, and they shift to 450 nm and 540 nm in alkaline solution (pH 8.50), respectively. An isobestic point at 440 nm (Fig. 2a) indicates the structural transformation of the probe A with increasing pH value. Similar results are observed for the probe B, revealing that both probes exhibit evident responses toward solution's acidity.

|
Download:
|
| Fig. 2. pH-dependent absorption (a) and emission (b) spectra of probe A, pH-dependent emission spectrum of probe B (c), and the fluorescence intensity at 529 nm of probe A/B as a function of pH (d). [A] = [B] = 10 μmol/L, λex = 440 nm. | |
The emission spectra of the probes A and B in media with different pH are shown in Figs. 2b and c, respectively. Figs. 2b and c illustrate that the fluorescence intensities of both probes increase with solution's acidity. The protonation of the appended tertiary amine hampers the PET process and the fluorescence is enhanced dramatically. Fig. 2d shows that when pH value changed from 9.58 to 6.51, the fluorescence intensity increased rapidly. The pKa values calculated for probes A and B were 7.60 and 7.38, respectively, which are close to the physiological pH, suggesting that both probes have the potential to be applied in biological systems.
The effect of particle size on the photophysical properties of the fluorescent nano-probes were investigated by absorption and emission spectra. The introduction of nano-particles to probes A and B hardly influences the absorption and emission maxima, while the fluorescence intensity decreases to some extent: The smaller the particle size is, the lower the fluorescence intensity becomes (Fig. 3a).

|
Download:
|
| Fig. 3. (a) Effect of particle size on the emission spectra of pH-A, (b) pH effect on the emission of pH-A (120 nm), and (c) plots of fluorescence intensity vs. pH. [pH-A] = 0.05 mg/mL, λex = 440 nm. | |
Then, the spectral responses of nano-probe pH-A with different sizes (20 nm, 120 nm and 300 nm) toward acidity were studied as well. Significant fluorescence increments but to different degrees for three kinds of nano-probes were obtained with the decrease in solution's pH. Fig. 3b shows the pH effect on the emission of pH-A with 120 nm. The maximum fluorescence enhancements for nanoprobes with particle sizes of 20 nm, 120 nm and 300 nm were 10- fold, 14-fold and 19-fold, respectively, all much lower than that of the probe A (~55-fold). From the plots of fluorescence intensity vs. pH (Fig. 3c), the pKa values for the above three nano-probes were respectively estimated to be 7.39, 7.45 and 7.80, which were close to the pKa of probe A. Whereas, the spectral responses of pH-B toward pH is quite different from that of pH-A. All nano-probe pHB with three different particle sizes displayed much smaller response to pH, probably due to the poor solubility and dispersion.
These results indicate that the introduction of nano-particles lowers the sensitivity of the probe to some extent. In the case of small particle size, spontaneous aggregation of nano-particles could happen, which led to relatively high local concentration of the probe and self-quenching in the fluorescence. However, the dispersibility of the nano-particles decreases with increasing particle size, which makes the measurement more difficult. Therefore, particles with 120 nm were selected in the following experiments.
Selectivity and competition are two important characters to evaluate the performance of the probes. Next, the effects of some common ions and small weighted thiols, including PO43-, NO3-, SO42-, NO2-, Cl-, SCN-, SO32-, HCO3-, Br-, S2-, AcO-, Cys, Hcy and GSH, on the fluorescence spectrum of pH-A were studied (Figs. 4 and S3 in Supporting information). The results reveal that other coexisted ions and thiols (>1000 equiv. of proton, Fig. S3 in Supporting information) induced neglected fluorescence changes of the nano-probe, suggesting that the probe exhibit high selectivity and good competition toward H+ over other biological relevant species.

|
Download:
|
| Fig. 4. The fluorescence intensity at 529 nm of pH-A with particle size of 120 nm in the presence of various analytes in 20 mmol/L PBS (a: pH 7.4; b: pH 4.0). [pH-A] = 0.5 mg/mL, [analytes]= 100 μmol/L, λex = 440 nm. | |
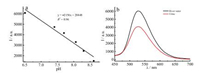
The fluorescence intensity of pH-A with particle size of 120 nm is linear with pH in the range of 6.51–8.61 (Fig. 5a), which is a bit narrower than that for probe A (6.58–9.50). In view of its high selectivity and good competition, this fluorescent nano-probe was employed to detect pH values of two realistic samples, water from Qingchun River in ECUST and human urine (Fig. 5b). The pH value of river water is calculated to be 6.57 (6.60 measured by pH meter) and that of human urine is 7.55 (7.60 by pH meter). The above results reveal that fluorescent nano-probe pH-A with particle size of 120 nm could be applied in the detection of pH in realistic samples.
|
Download:
|
| Fig. 5. (a) Linear relationship between fluorescence intensity at 529 nm and pH, and (b) the emission spectra of pH-A (120 nm) in river and urine. [pH-A] = 0.05 mg/mL, λex = 440 nm. | |
In conclusion, fluorescent nano-probes for proton were prepared by covalently binding two small weighted fluorescent probes to the surface of SiO2 NPs with particle sizes of 20 nm, 120 nm and 300 nm through click reaction. The results showed that the photophysical properties of the nano-probes were mainly affected by the particle size. In view of the aggregation and the dispersion of the nano-probes, that with particle size of 120 nm was selected to measure the pH in realistic samples. This work could provide some new information for the design of fluorescent nano-probes.
AcknowledgmentThe authors acknowledge financial support from the National Natural Science Foundation of China (No. 21576085).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.07.018.
| [1] |
J. Han, K. Burgess, Chem. Rev. 110 (2009) 2709-2728. |
| [2] |
Q.Z. Jia, C. Wang, X.N. Du, et al., Chin. Pharmacol. Bull. 21 (2005) 82-87. |
| [3] |
C. Hille, M. Berg, L. Breeerl, et al., Anal. Bioanal. Chem. 391 (2008) 1871-1879. DOI:10.1007/s00216-008-2147-0 |
| [4] |
S. Charier, O. Ruel, J.B. Baudin, et al., Angew. Chem. Int. Ed. 43 (2004) 4785-4788. |
| [5] |
S.T. Hong, T.H. Kim, W. J.-Choi, et al., Anal. Chem. 89 (2017) 9830-9835. DOI:10.1021/acs.analchem.7b01804 |
| [6] |
T. Mathew, P.H. Yin, Y. Gary, J. Am. Chem. Soc. 133 (2011) 10034-10037. DOI:10.1021/ja202902d |
| [7] |
J. Guo, L. Qiu, Z. Deng, F. Yan, Polym. Chem. 4 (2013) 1309-1312. DOI:10.1039/c2py21076b |
| [8] |
A.P. de Silva, S.S.K. de Silva, N.C.W. Goonesekera, et al., J. Am. Chem. Soc. 129 (2007) 3050-3051. DOI:10.1021/ja0686514 |
| [9] |
L. Song, X.D. Sun, Y. Ge, et al., Chin. Chem. Lett. 27 (2016) 1776-1780. DOI:10.1016/j.cclet.2016.05.007 |
| [10] |
Z. Chen, Q. Sun, Y. Yao, et al., Biosens. Bioelectron. 91 (2017) 553-559. DOI:10.1016/j.bios.2017.01.013 |
| [11] |
Y. Chen, T. Wei, Z. Zhang, et al., Chin. Chem. Lett. 28 (2017) 1957-1960. DOI:10.1016/j.cclet.2017.05.010 |
| [12] |
C. Zong, K. Ai, G. Zhang, H. Li, L. Lu, Anal. Chem. 83 (2011) 3126-3132. DOI:10.1021/ac2001324 |
| [13] |
C.S. Lim, S.T. Hong, S.S. Ryu, D.E. Kang, B.R. Cho, Chem. Asian J. 10 (2015) 2240-2249. DOI:10.1002/asia.201500314 |
| [14] |
J. Yao, M. Yang, Y. Duan, Chem. Rev. 114 (2014) 6130-6178. DOI:10.1021/cr200359p |
| [15] |
Y.H. Jin, S. Kannan, M. Wu, et al., Chem. Res. Toxicol. 20 (2007) 1126-1133. DOI:10.1021/tx7001959 |
| [16] |
R.K. Sharma, S. Das, A.J. Maitra, Colloid Interface Sci. 277 (2004) 342-346. DOI:10.1016/j.jcis.2004.04.019 |
| [17] |
S. Das, T.K. Jain, A. Maitra, J. Colloid Interface Sci. 252 (2002) 82-88. DOI:10.1006/jcis.2002.8404 |
| [18] |
A.B. Chinen, C.M. Guan, J.R. Ferrer, et al., Chem. Rev. 115 (2015) 10530-10574. DOI:10.1021/acs.chemrev.5b00321 |
| [19] |
D. Wu, Y.Z. Shen, J.H. Chen, et al., Chin. Chem. Lett. 28 (2017) 1979-1982. DOI:10.1016/j.cclet.2017.07.004 |
| [20] |
Y.Y. He, Z.X. Li, Q.Y. Jia, et al., Chin. Chem. Lett. 28 (2017) 1969-1974. DOI:10.1016/j.cclet.2017.07.027 |
| [21] |
Y. Kang, J.L. Fan, Q. Jin, et al., Chin. Chem. Lett. 28 (2017) 1991-1993. DOI:10.1016/j.cclet.2017.08.054 |
| [22] |
J. Wen, P.Y. Xia, Z.M. Zheng, et al., Chin. Chem. Lett. 28 (2017) 2005-2008. DOI:10.1016/j.cclet.2017.09.014 |
| [23] |
H.I. Un, S. Wu, C.B. Huang, Z. Xu, L. Xu, Chem. Commun. 51 (2015) 3143-3146. DOI:10.1039/C4CC09488C |
| [24] |
L. Song, T. Jia, W.J. Lu, et al., Org. Biomol. Chem. 12 (2014) 8422-8427. DOI:10.1039/C4OB01219D |
 2018, Vol. 29
2018, Vol. 29