b Key Laboratory of Pesticide and Chemical Biology, Ministry of Education College of Chemistry, Central China Normal University, Wuhan 430079, China
Mechanochromic material, as one of stimuli-responsive smart materials, has drawn much attention due to their potential application in many fields such as security, stress sensing and information storage [1-6]. In recent years, some representative molecular backbones were consecutively reported. For instance, Tian et al. developed the anthracene-based vinyl pyridine, which displayed three types of aggregation state by single crystal structure and accompanied by different emission [7]. Xu and Chi found that the carbazole-modified sulphone had fluorescentphosphorescent dual emission upon the mechanical stimuli [8]. Fraser et al. reported the mechanochromic difluoroboronavobenzone [9]. Our group also developed a series of isocyano-based gold complexes with mechanochromic behavior [10-17]. Except for that, tetraphenylene (TPE) as the classic molecular system has also been widely applied in the construction of mechanochromic materials [18]. And all kinds of conjugated bridges are employed to participate in the molecular architecture with mechanochromic manner. For example, vinyl-bridge can be used as an efficient conjugated unit to prolong the molecular conjugation by a Heck coupling reaction [19, 20]. Two vinyl-bridge modified TPE compounds with extended conjugation were synthesized, and their photophysical properties and mechanochromic performance were investigated.
Two TPE-based derivatives TPE-Py and TPE-Ph were synthesized according to the method outlined in Scheme 1. As shown, 2- vinylpyridine and 4-nitrostyrene were selected as the vinyl bridges to carry out the Pd-catalyzed Heck coupling reaction with tetrabrominated TPE [21], affording the targeting molecules TPE-Py and TPE-Ph in 20.6%-13.1% yields. Their structures have been welldefined by NMR and mass spectrometry. Fortunately, we also obtained the single crystal structure of TPE-Py. A single crystal suitable for crystallographic analysis was obtained by slow diffusion of hexane into a tetrahydrofuran solution of TPE-Py. From the crystal structure (Fig. S1 in Supporting information), TPE-Py showed a similar structural configuration with the literature owing to similar intermolecular multi weak interactions such as C-H…π and C-H…N hydrogen bonds, implying that it can respond to the external stimuli [20].

|
Download:
|
| Scheme 1. The synthesis of mechanochromic TPE-Py and TPE-Ph. | |
To gain insight into the geometry and electronic behavior of TPE-Py and TPE-Ph, time-dependent density functional theory (TD-DFT) calculations were carried out at the B3LYP/6-31G* level with the Gaussian 09 program to clarify its frontier molecular orbitals and electronic transitions. These TD-DFT calculations prospected four possible absorption wavelengths for TPE-Py at around 478 nm, 415 nm, 373 nm and 330 nm. The electron density of HOMO was mainly distributed on the TPE moiety, but at the first transition (HOMO→LUMO) the electron density shifted across the vinyl bridge to the pyridine groups whereas the electron density was more delocalized in the whole molecular at the second transition (HOMO→LUMO+1). The third transition (HOMO- 2→LUMO) presented the electron density tended to gather in the vinyl of TPE on the contrast of the fourth transition (HOMO- 1→LUMO +2). Analogously, TPE-Ph also was predicted four intense absorption transition according to the TD-DFT result. No obvious charge transfer was observed in HOMO-2 and LUMO orbitals. However, the electron density was located on the nitrobenzene unit in LUMO +2 orbital in comparison to the LUMO orbital. The detailed information was outlined in Fig. 1.

|
Download:
|
| Fig. 1. HOMO and LUMO frontier molecular orbitals of TPE-Py and TPE-Ph and obtained at the DFT level using a B3LYP/6-31 G (d, p) basis set. | |
Owing to AIE (aggregation-induced emission) character of TPE, we firstly investigated their AIE properties. The fluorescence spectra of TPE-Py and TPE-Ph in H2O/THF gradient solution are checked. As shown in Fig. 2A, TPE-Py in THF solution showed a weak emission at 545 nm. With the volume fraction of water increasing to 80%, the fluorescence intensity gradually increased, which was probably attributed to the formation of aggregated state. And the aggregation can restrict the rotation of phenyl groups, and block the radiationless relaxation as a result of the enhancement of fluorescence intensity. The result was similar to our previous report [18]. However, when the volume fraction of water further increased to 90% as shown in Fig. 2B, the fluorescence showed a quenching trend and accompanied by a little blue shift owing to emission decreasing in high concentration. More compact nano-particles might form during this process, which probably led to Mie scattering and decreasing fluorescence. These results were well in agreement with a previous report [20]. However, in comparison to the pyridinecoating TPE-Py, the fluorescence intensity of vinylnitrobenzenecontaining TPE-Ph quickly decreased when the water fraction was only upon to 10%, possibly due to the fact that TPE-Py involved in the twisted intra-molecular charge transfer (TICT) [22]. With the water fraction increasing to 50%, similar increasing emission was also observed as shown in Fig. 2C, and the reason could be assumed to the restriction of intramolecular rotations (RIR) of aggregation state, as presented in Fig. 2D. Subsequently, we investigated the influence of different solvents for UV–vis absorption and fluorescence spectra at the same concentration of 10-5 mol/L. In comparison to the few changes of UV–vis absorption, the fluorescence spectrum of TPE-Py displayed obvious red shift from 540 nm to 580nm with decreasing solvent polarity (Fig. S2 in Supporting information). For compound TPE-Ph, along with the enhancement of solvent polarity, the UV–vis absorption exhibited a few redshift while the corresponding fluorescence intensity gradually decreased (Fig. S3 in Supporting information). Especially, compound TPE-Ph showed the strongest fluorescent emission in nonpolar CCl4. To verify the influence of RIR (restriction of intramolecular rotation) to the fluorescence manner, the viscosity-dependent fluorescence spectrum was measured. In methanol-glycerol solution, the fluorescence intensity gradually increased with the enhancement of glycerol volume fraction (Fig. S4 in Supporting information), owing to the fact that larger viscosity restricts the molecule rotation and reduces nonradiative transition energy loss.

|
Download:
|
| Fig. 2. Fluorescence spectra of TPE-Py (A) (10 mmol/L; excited wavelength: 340 nm) and TPE-Ph (B) (10 mmol/L; excited wavelength: 390 nm) at different water fractions (0-90%, v/v) in H2O/THF mixture. A plot of the maximum fluorescence intensity against the water fraction of TPE-Py (C) and TPE-Ph (D). | |
Next, we studied the optical behavior in solid state. According to the UV–vis absorption spectra of compounds TPE-Py and TPE-Ph, no obvious changes were observed (Fig. S5 in Supporting information). In fluorescence spectra of TPE-Py, the asprepared form exhibited a bright green emission at 520 [35_TD$DIFF]nm, which could shift to 560 nm upon grinding and companied by the color changes from green to orange in Fig. 3A. In addition, the ground powder could transfer to the original fluorescence by fuming it with dichloromethane vapor. Similarly, TPE-Ph presented bright jacinth fluorescence before grinding with a maximal emission peak at 605 nm.
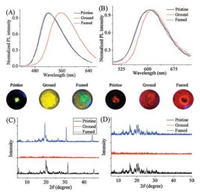
|
Download:
|
| Fig. 3. Normalized fluorescence emission spectra of TPE-Py (A) and TPE-Ph (B) at pristine, ground and fumed states; The emission images of luminophor respectively under UV illumination were below the spectra. The PXRD spectra of TPE-Py (C) and TPE-Ph (D). | |
After grinding, the fluorescence maximum shifted to 618 nm, and a simple fuming with dichloromethane promoted it return to theinitialstate, aspresentedin Fig. 3B. These observations strongly indicated that compounds TPE-Py and TPE-Ph had reversible mechanofluorochromic property.
To evaluate the aggregated states of TPE-Py and TPE-Ph, we measured their powder X-ray diffraction (PXRD) in pristine, ground and fumed states. As shown in Figs. 3C and D, the pristine TPE-Py and TPE-Ph exhibited obvious sharp diffraction peaks, which implied they were in the crystalline phase. After grinding, the fact of the disappeared diffraction peaks suggested that they had changed from crystal forms to irregular amorphous forms. Upon exposing the ground powder with dichloromethane vapor, almost all of identical peaks reappeared, suggesting that the amorphous powder returned to crystalline. The result of PXRD attested that the mechanical stimuli can induce the changes of aggregation and emission, possibly ascribing to the enhanced intramolecular coplanarity of the twisted TPE derivatives in the process of crystal forms to irregular amorphous forms [23].
In conclusion, two symmetric conjugated TPE derivatives TPE-Py and TPE-Ph were synthesized by Heck coupling reaction. We investigated their AIE behaviors and mechanochromic properties. The results in solution show that they have good AIE property. The performance in solid state presented that the mechanical stimuli led to 13–40 nm red-shift, and could return to the original state by solvent fuming. The above research is meaningful to future AIE active mechanofluorochromic materials development in a wide range of bioimaging and safety applications.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21676113, 21402057, 21772054), Distinguished Young Scholar of Hubei Province (No. 2018CFA079), Youth Chen-Guang Project of Wuhan (No. 2016070204010098), the 111 Project (No. B17019), the Ministry-Province Jointly Constructed Base for State Key Lab-Shenzhen Key Laboratory of Chemical Biology (Shenzhen), the State Key Laboratory of Materials-Oriented Chemical Engineering (No. KL17-10), the Open Project Fund of Key Laboratory of Natural Resources of Changbai Mountain & Functional Molecules, Yanbian University, Ministry of Education (No. NRFM201701), the Foundation of Key Laboratory of Synthetic and Biological Colloids, Jiangnan University, Ministry of Education (No. JDSJ2017-07), Self-determined Research Funds of CCNU from the Colleges' Basic Research and Operation of MOE (No. CCNU18TS012).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.06.022.
| [1] |
(a) X.Q. Zhang, Z.G. Chi, Y. Zhang, S.W. Liu, J.R. Xu, J. Mater. Chem. C 1 (2013) 3376-3390; (b)R. Gao, Y.B. Zhao, X.G. Yang, D.P. Yan, RSC Adv. 5 (2015) 56470-56477. |
| [2] |
(a) S.F. Xue, X. Qiu, Q.K. Suna, W.J. Yang, J. Mater. Chem. C 4 (2016) 1568-1578; (b)L. Wang, K.Q. Ye, H.Y. Zhang, Chin. Chem. Lett. 27 (2016) 1367-1375. |
| [3] |
(a) P. Zhang, Z.Q. Guo, C.X. Yan, W.H. Zhu, Chin. Chem. Lett. 28 (2017) 1952-1956; (b)P.C. Xue, J.P. Ding, P.P. Wang, R. Lua, J. Mater. Chem. C 4 (2016) 6688-6706; (c)Y. Li, C. Xu, C. Shu, X. Hou, P. Wu, Chin. Chem. Lett. 28 (2017) 1961-1964; (d)C. Wang, Z. Li, Mater. Chem. Front. 1 (2017) 2174-2194. |
| [4] |
(a) D. Wu, Y. Shen, J. Chen, et al., Chin. Chem. Lett. 28 (2017) 1979-1982; (b)Z. Xu, J. Chen, L.L. Hu, et al., Chin. Chem. Lett. 28 (2017) 1935-1942. |
| [5] |
(a) F. Song, R. Liang, J. Deng, Z. Liu, X. Peng, Chin. Chem. Lett. 28 (2017) 1997-2000; (b)S.Z. Li, Y.J. Lin, D.P. Yan, J. Mater. Chem. C 4 (2016) 2527-2534; (c)G.L. Fan, D.Y. Peng, Adv.Opt. Mater. 4 (2016) 2139. |
| [6] |
(a) R. Zhang, Q. Wang, X. Zheng, J. Mater. Chem. C 6 (2018) 3182-3199; (b)D.P. Yan, H.J. Yang, Q.Y. Meng, H.Y. Lin, M. Wei, Adv. Funct. Mater. 24 (2014) 587. |
| [7] |
Y.J. Dong, B. Xu, J.B. Zhang, et al., Angew. Chem. Int. Ed. 51 (2012) 10782-10785. DOI:10.1002/anie.v51.43 |
| [8] |
(a) S. Leng, Q.L. Qiao, Y. Gao, et al., Chin. Chem. Lett. 28 (2017) 1911-1915; (b)Y. Chen, T. Wei, Z. Zhang, et al., Chin. Chem. Lett. 28 (2017) 1957-1960; (c)Z. Mao, Z.Y. Yang, Y.X. Mu, et al., Angew. Chem. Int. Ed. 54 (2015) 6270-6273. |
| [9] |
G.Q. Zhang, J.W. Lu, M. Sabat, C.L. Fraser, J. Am. Chem. Soc. 132 (2010) 2160-2162. DOI:10.1021/ja9097719 |
| [10] |
J.H. Liang, Z. Chen, L.J. Xu, et al., J. Mater. Chem. C 2 (2014) 2243-2250. DOI:10.1039/c3tc31638f |
| [11] |
Z. Chen, J. Zhang, M. Song, et al., Chem. Commun. 51 (2015) 326-329. DOI:10.1039/C4CC08087D |
| [12] |
Z. Chen, Z. Li, L. Yang, et al., Dyes Pigments 121 (2015) 170-177. DOI:10.1016/j.dyepig.2015.05.021 |
| [13] |
Z. Chen, J.H. Liang, Y.T. Nie, et al., Dalton Trans. 44 (2015) 17473-17477. DOI:10.1039/C5DT02035B |
| [14] |
Z. Chen, L. Yang, Y.X. Hu, et al., RSC Adv. 5 (2015) 93757-93764. DOI:10.1039/C5RA19378H |
| [15] |
Z. Chen, Z. Li, F. Hu, et al., Dyes Pigments 125 (2016) 169-178. DOI:10.1016/j.dyepig.2015.10.038 |
| [16] |
J. Zhang, Z. Chen, L. Yang, et al., Dyes Pigments 136 (2017) 168-174. DOI:10.1016/j.dyepig.2016.08.051 |
| [17] |
Y.B. Dong, Z. Chen, L. Yang, et al., Dyes Pigments 150 (2018) 315-322. DOI:10.1016/j.dyepig.2017.12.030 |
| [18] |
(a) X. Han, B.B. Zhang, J.H. Chen, et al., J. Mater. Chem. B 5 (2017) 5096-5100; (b)L. Wang, K.Q. Ye, H.Y. Zhang, Chin. Chem. Lett. 27 (2016) 1367-1375. |
| [19] |
J.H. Chen, D.Y. Li, W.J. Chi, et al., Chem.-Eur. J. 24 (2018) 3671-3676. DOI:10.1002/chem.201705780 |
| [20] |
J.B. Xiong, K. Wang, Z.Q. Yao, et al., ACS Appl. Mater. Interfaces 10 (2018) 5819-5827. DOI:10.1021/acsami.7b18718 |
| [21] |
X.F. Duan, J. Zeng, J.W. Lv, Z.B. Zhang, J. Org. Chem. 71 (2006) 9873. DOI:10.1021/jo061644d |
| [22] |
Q.K. Qi, J.Y. Qian, X. Tan, et al., Adv. Funct. Mater. 25 (2015) 4005-4010. DOI:10.1002/adfm.v25.26 |
| [23] |
(a) D.P. Yan, D.G. Evans, Mater. Horiz. 1 (2014) 46-57; (b)Q.K. Qi, Y.F. Liu, X.F. Fang, et al., RSC Adv. 3 (2013) 7996-8002; (c)Q.K. Qi, J.B. Zhang, B. Xu, et al., J. Phys. Chem. C 117 (2013) 24997-25003. |
 2018, Vol. 29
2018, Vol. 29 


