b Zhangjiang Institute, China State Institute of Pharmaceutical Industry, Shanghai 201203, China
Carbon dioxide (CO2) is one of the catabolic end products of cellular respiration in organisms that gain energy by decomposing sugars, fats and amino acids with oxygen [1]. Moreover, as a very common component of gas mixtures from several natural and anthropogenic processes including the combustion of fossil fuels for power generation, natural gas production, oil refining, and most chemical manufacturing, carbon dioxide has significant impacts on globe climate and human wellbeing such as the greenhouse effect, rise in sea levels, and probable expansion of subtropical deserts as well [2-8]. Recent research suggests that the carbon dioxide concentrations in cells are closely related to multiple physiological and biological processes such as respiratory regulation, pH equilibrium in the blood, carcinogenesis, genetic material replication, nucleic acid base formation, and cell proliferation [9]. Therefore, the development of highly efficient methods for the detection and monitoring of CO2 is extremely important in widespread fields such as chemical, environmental monitoring, clinical analysis, and agri-food industry [10, 11].
Many approaches, such as infrared spectroscopic technique, electrochemical assay, gas chromatography-mass spectrometry (GC-MS) technique, field-effect transistors have been employed to measure and monitor CO2 [12-14]. However, these methods often suffered from high cost, time-consuming processes, and lack of temporal and spatial resolution. Due to its simple, inexpensive, rapid sensing, and real-time detection abilities, fluorescence technique has proven to be one of the most efficient approaches for the detection of CO2 [15-23].
During thepastfewyears, avarietyofsmall-moleculefluorescent probes for the selective detection of CO2 have been developed [24-29]. For example, Bouffard and Yoon et al. prepared a polydiacetylene-based colorimetric and fluorescent turn-on sensor for the selective sensing of CO2 with high sensitivity [30]. Although the preparation of CO2 fluorescent probes has attracted increasing attentions, surprisingly, there have been few reviews in the literature on the description of the development of fluorescent probes for the selective detection of CO2 [31]. Considering the fact that many important progress has been made during the last few years, it is time to summarize the recent advances in the preparation of such fluorescent probes. In this review, we will highlight the recent advances in the design and preparation of small-molecule fluorescent probes for the selective detection and monitoring of CO2. Moreover, their properties and functions will be discussed as well. In order to make it reasonable and easy to understand, in this review, these fluorescent probes will be classified and discussed depending on their sensing mechanisms.
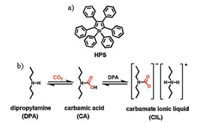
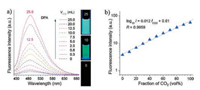
2. Aggregate-induced emission (AIE)-based small-molecule fluorescent probes for the detection of CO2As an aggregation-induced emission (AIE) chromophore, hexaphenylsilole (HPS), which displayed nonluminescent in solutions but highly emissive as aggregates, was discovered in Tang's pioneering work [32, 33]. It has been certified that restriction of intramolecular rotations (RIR) of its multiple phenyl rotors in the aggregate was responsible for the AIE effect [34]. Therefore, purging an amine solution of HPS with a stream of CO2 gas may turn on the fluorescence emission of HPS since bubbling CO2 through an amine can yield a carbamate ionic liquid (CIL), which induces the increases in polarity and viscosity thereby activating the RIR process of HPS (Scheme 1). Recently, Tang et al. developed an AIE-based fluorescent probe HPS (herein, also named as 1) for the detection of CO2 [35]. As shown in Fig. 1a, when the dipropylamine (DPA) solution of HPS was bubbled with increasing volumes of CO2, the emission intensity of the spectra feature at around 480 nm was seen to increase and a very bright green light was observed. Moreover, a linear relationship was found between the fluorescence intensity at around 480 nm and the fraction of CO2 (fCO2) indicated probe HPS could detect CO2 quantitatively (Fig. 1b). The sensing mechanism of probe HPS for CO2 was verified by spectroscopic and microscopic analyses, NMR and IR spectra, and transmission electron microscope (TEM). In this work, the authors developed a simple fluorescent probe for visualizing the presence of CO2 and quantitative determination of its amount over the whole concentration range (0-100%).
|
Download:
|
| Scheme 1. (a) The strcuture of 1, 1, 2, 3, 4, 5-hexaphenylsilole (HPS). (b) Formation of carbamate ionic liquid (CIL) by bubbling CO2 gas through dipropylamine (DPA) liquid. | |
|
Download:
|
| Fig. 1. (a) Fluorescence spectra and photographs of HPS (1) in DPA before and after bubbling with different volumes of CO2 (VCO2). All the photographs were taken under UV illumination. (b) Plots of fluorescence intensities (Ⅰ) of HPS (1) versus fractions of CO2 (fCO2) in CO2/N2 mixtures. Copied with permission [35]. Copyright 2015, American Chemical Society. | |
Based on the similar strategy, Tong and Dong et al. designed and synthesized a novel fluorescent probe 2 derived from the 1, 2, 5-triphenylpyrrole (TPP) core containing many tertiary amine moieties (Fig. 2) [36]. For probe 2, it contained three tertiary amine moieties those can be easily neutralized to a more water soluble ammonium salt by bubbling of carbon dioxide, thus, the fluorescence of 2 would be quenched as a result of disaggregation. As shown in Fig. 3a, upon bubbling with a low amount of carbon dioxide, the fluorescent intensity of 2 was gradually reduced. The relationship between the changes in emission intensity and the volume of carbon dioxide indicated that this approach was applicable for the quantitative detection of low amount of carbon dioxide (Fig. 3b). Then, the quantitative detection of the fraction of CO2 (fCO2) in a gas mixture was conducted by employing CO2-air mixtures as model systems. As shown in Fig. 4a, the fluorescent intensity of 2 ((I-I0)/I0) decreased linearly with increasing amounts of fCO2 in the range from 0.031% to 5%. Interestingly, upon repeated bubbling of CO2 and addition of 0.33 mmol/L NaOH solution, the emission of 2 showed reversible on-off changes, which suggested that the probe 2 was able to detect CO2 reversibly (Fig. 4b). More importantly, probe 2 exhibited high selectivity to CO2 as witnessed by the fact that the addition of N2, O2, CH4, Ar, and air to the solution of 2 induced negligible changes in the emission while the addition of CO2 caused a significant decrease of fluorescence. The detection mechanism was confirmed by UV-vis spectroscopy and dynamic light scattering (DLS) analyses. The results showed that ammonium salts was generated through the reaction of CO2 (carbonic acid) with the tertiary amine when CO2 was added by bubbling into the solution of 2. So, the probe 2 aggregates gradually dissolved or disassembled in the mixture as evidenced by the increase of the transmittance and the decrease of the particle diameter size, which led to the quenching of the fluorescence of 2. Therefore, this work provided a facile, rapid, and naked-eyediscernable method for the detection of CO2 with high sensitivity, selectivity and reversibility.

|
Download:
|
| Fig. 2. The strcuture of probe 2 and fluorescence detection of a low amount of CO2 with probe 2 in THF-water mixtures. Reproduced with permission [36]. Copyright 2015, Royal Society of Chemistry. | |

|
Download:
|
| Fig. 3. (a) Fluorescence titration spectra of probe 2 (100 μL) in THF/H2O (1/9, v/v, 3 mL) upon bubbling with different volumes of CO2. Inset: photographs of probe 2 in the absence and presence of CO2. The photographs are taken under UV illumination. (b) Emission intensities of probe 2 as a function of [CO2] or [CO2]2 (0-81 μL). Excitation wavelength: 300 nm. Copied with permission [36]. Copyright 2015, Royal Society of Chemistry. | |

|
Download:
|
| Fig. 4. (a) Plots of emission intensities ((I-I0)/I0) of probe 2 versus fraction of CO2-air mixtures. (b) Maximum emission intensity change of 2 (100 μmol/L) in the THF/ H2O (1/9, v/v, 3 mL) mixture upon bubbling with 0.5 mL of carbon dioxide (bottom) and addition of 0.33 mmol/L NaOH (top) versus repeating cycles. Copied with permission [36]. Copyright 2015, Royal Society of Chemistry. | |
3. Anion-activated small-molecule fluorescent probes for the detection of CO2
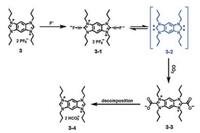
It has been reported that N-heterocyclic carbenes (NHCs), which are often produced by deprotonating the corresponding imidazolium salts, has an intrinsic ability to activate CO2 to form imidazolium carboxylates [37]. Recently, Sessler, Yoon, Lee et al. designed and synthesized a specific NHC precursor tetrapropyl benzobisimidazolium salts (3), which could be activated by fluoride to generate an N-heterocyclic carbene intermediate, as a novel probe for the fluorescent and colorimetric detection of CO2 (Scheme 2) [38]. Preliminary studies showed that the addition of F- ion (added as the tetrabutylammonium salt, TBAF) induced red shift of the absorption band from 290 nm to 344 nm as well as an efficient fluorescence quenching. Moreover, probe 3 displayed a high selectivity for the fluoride anion over other halide anions such as Cl-, Br-, and I-. Then, the response of the solution of probe 3 containing F- to CO2 was carried out. As shown in Fig. 5, when the solution obtained by treatment with 3.0 equiv. of TBAF was bubbled with increasing volumes of CO2, a blue shift of the absorption band from 344 nm to 290 nm with an isosbestic point localized at 312 nm was observed, correspondingly, a significant increase in the fluorescence intensity was observed as well. The results showed that the spectroscopic changes were strongly dependent on the CO2 concentration and the CO2 detection limit of this system was determined to be ca. 30 ppm. The sensing mechanism was investigated by 1H, 13C and 19F NMR spectra and density functional theory (DFT) analyses, which provided rigorous evidence that the sensing mechanism was ascribed to the fluoride-induced formation of an N-heterocyclic carbene intermediate that reacted with CO2 to form an imidazolium carboxylate.
|
Download:
|
| Scheme 2. Proposed reactions that occur when probe 3 is treated first with F- and then CO2. | |

|
Download:
|
| Fig. 5. (a) UV spectra of probe 3 (15.0 μmol/L, 3.0 mL) recorded in CH3CN in the presence of TBAF (3.0 equiv. of F-) and then bubbling with different volumes of CO2. (b) Changes in the fluorescence titration spectral features of probe 3 (15.0 μmol/L) in CH3CN observed upon the addition of TBAF (3.0 equiv.) and then bubbling with different volumes of CO2 with excitation at 290 nm. Inset: plot of the fluorescence intensity I344nm of a solution consisting of probe 3 and 3.0 equiv. of TBAF versus various volumes of CO2 with excitation effected at 290 nm. Reproduced with permission [38]. Copyright 2012, American Chemical Society. | |
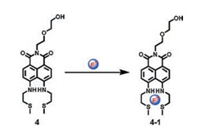
Xu and Yoon et al. prepared a new naphthalimide derivative 4 as an F--activated CO2 sensor (Scheme 3) [39]. Probe 4 exhibited selective colorimetric and fluorescence changes with F-. For example, the addition of F- into the solution of probe 4 caused redshift of the absorption spectra and an obvious fluorescence quenching of probe 4, which was attributed to the strong hydrogen bonding between amine groups and anions. Then, the complex (probe 4 in the presence of 4 equiv. of F-) was used to detect CO2 gas. As shown in Fig. 6, upon the addition of CO2, an observable blue-shift of the absorption spectra and the fluorescence enhancements were observed. Moreover, the addition of CO2 induced the color of solution change from dark yellow to pale yellow. The detection limit of the complex (probe 4 in the presence of 4 equiv. of F-) for CO2 was calculated to be 2.04 ×10-7 mol/L. Therefore, a novel fluorescent and colorimetric sensor 4 for CO2 was developed.
|
Download:
|
| Scheme 3. The strcuture of probe 4. | |

|
Download:
|
| Fig. 6. (a) UV-vis and (b) fluorescence emission spectral changes of probe 4 (10 μmol/L) upon bubbling with different volumes of CO2 in the presence of 4 equiv. of F-. Inset: (a) Photograph showing the color changes corresponding to the UV spectral changes; (b) Photograph showing the color changes corresponding to the fluorescence emission spectral changes. Reproduced with permission [39]. Copyright 2015, Elsevier Ltd. | |
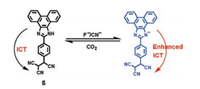
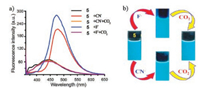
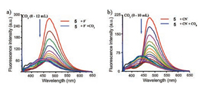
More recently, based on the similar sensing mechanism, Misra et al. developed an efficient donor-acceptor type intramolecular charge transfer (ICT) probe tricyanoethylphenyl phenanthroimidazole 5 for the detection of CO2 (Scheme 4) [40]. The complexes (probe 5 in the presence of F- or CN-) were applied to sense CO2 gas since an anionic nitrogen of probe 5 could be in situ generated in the presence of F- and CN- anions. Figs. 7 and 8 indicated that the emission intensity centered at 475 nm diminished completely along with revival in the original color of the probe solution by bubbling of the increasing volume of CO2 to the individual solution of in situ generated 5 + F- and 5 + CN- system, respectively. The detection limit of 5 + F- and 5 + CN- system for CO2 was estimated to be 1.75 ×10-6 mol/L (~77 ppb) and 1.06 ×10-6 mol/L (~47 ppb), respectively. The decrease in emission intensity of 5 + F- and 5 + CN- system with the addition of CO2 was mainly due to the formation of imidazolium hydrogen carbonate salt, which was formed from the decomposition of an unstable N-CO2 adduct generated from initial attack of negatively charged imidazolium ion and CO2.
|
Download:
|
| Scheme 4. The strcuture of probe 5 and proposed mechanism of interaction of 5 with F-/CN- ions. | |
|
Download:
|
| Fig. 7. (a) Change in emission spectra of probe 5 (1 μmol/L) upon addition of F- (100 equiv.) and CN- (125 equiv.) followed by the addition of CO2 (excess) in MeCN (λex = 355 nm). (b) Image shows respective color change of probe 5 with F-/CN- and then CO2. Reproduced with permission [40]. Copyright 2016, Elsevier Ltd. | |
|
Download:
|
| Fig. 8. Changes in emission spectra of probe 5 (1 μmol/L) upon titration of (a) 100 equiv. of F- and (b) 125 equiv. of CN- followed by the addition of CO2 in MeCN (λex = 355 nm). Reproduced with permission [36]. Copyright 2016, Elsevier Ltd. | |
4. Intra-molecular hydrogen bond-based small-molecule fluorescent probes for the detection of CO2
It has been reported that amino acids and their derivatives have the ability to react with CO2 to form carbamic acids [41]. Thus, amino acids and their derivatives have been widely developed as the recognition receptors for CO2 [42, 43]. However, the instability of carbamic acids caused the prepared probe displayed unsatisfactory selectivity and sensitivity for CO2. With the aim of stabilizing the unstable carbamic acid, additional functional groups could be introduced to bind with carbamic acid to generate an intramolecular hydrogen bond.
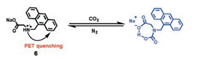
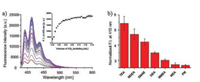
Han and co-workers reported an amino acid based fluorescent CO2 sensor 6, in which the amine reacted with CO2 to form a carbamic acid thereby an intramolecular hydrogen bond was formed with carbamic acid as proton donor and carboxylate anion as proton acceptor (Scheme 5) [44]. Sensor 6 displayed weak fluorescence emission due to the photoinduced electron transfer (PET) from the amine moiety to the anthracene [45-48]. However, in the presence of CO2, the reaction of 6 with CO2 resulted in the formation of a stable carbamic acid adduct through an intramolecular hydrogen bond with the carboxylate anion in 6, which blocked the PET process and induced a fluorescence enhancement of the sensor 6. As shown in Fig. 9a, upon addition of CO2 into the solution of 6, a dramatic increase in the fluorescence of 6 was observed. Moreover, the fluorescence intensity of 6 at 415 nm was almost proportional to the increasing volume of CO2. The detection limit of probe 6 for CO2 was calculated to be ca. 2 ppm based on the fluorescence spectra, which suggested that probe 6 was one of the most sensitive fluorescent CO2 chemosensors. Controlled experiments indicated that the high sensitivity of probe 6 for CO2 was attributed to the formation of an intramolecular hydrogen bond between the carbamic acid in the chemosensor-CO2 adduct acting as a hydrogen donor and the carboxylate in the chemosensor acting as a hydrogen acceptor. Furthermore, the results also demonstrated that the carboxylate moiety in 6 played a significant role in detecting CO2. The investigation of the reversible fluorescence changes of 6, which was conducted by alternatively bubbling CO2 and nitrogen into the solution of probe 6, showed that the fluorescence of 6 increased when CO2 was added while the enhanced fluorescence of 6 reverted to the original states when N2 was added. These results suggested that the repeated fluorescence changes of 6 were fully reversible.
|
Download:
|
| Scheme 5. Schematic illustration of probe 6 based CO2 sensing. | |
|
Download:
|
| Fig. 9. (a) Fluorescence spectra changes of probe 6 (50 μmol/L, 3 mL) in EtOH after bubbling with different volumes of CO2 (λex = 365 nm). Inset: Plot of fluorescence intensity at 415 nm versus volume of CO2 bubbling. (b) Normalized fluorescence intensities of probe 6 (50 μmol/L, 3 mL) after CO2 bubbling (0.048 mL) in EtOH with varying mixed CO2-absorbents (5 mmol/L). Copied with permission [44]. Copyright 2015, Elsevier Ltd. | |
Finally, the application of probe 6 as an easy screening protocol for evaluating the relative efficiency of CO2 absorbents was investigated. Correlated with previous reported results, the capturing tendency of CO2 absorbents screened by probe 6 was found to be 2-piperidinemetanol (PM), monoethanolamine (MEA), and N-methylethanolamine (MMEA) > diethanolamine (DEA) and dimethyletanolamine (DMEA) > N-methyldiethanolamine (MDEA) and triethanolamine (TEA) (Fig. 9b). Thus, probe 6 not only can detect CO2 with high sensitivity but also has the potential application for high throughput screening (HTS) to determine strong CO2 absorbents from a large number of CO2 absorbent candidates.
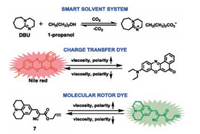
5. Multidye-based small-molecule fluorescent probes for the detection of CO2It has been proved that the reaction of some secondary/tertiary amines with CO2 gasinthe presence of alcohols produces carbonate/carbamate ionicliquids, which causes their polarityand viscosityare significantly changed to higher values [49]. Moreover, it is wellknown that polaritychanges and viscositychanges canbe visualized by charge transfer dyes and molecular rotor dyes, respectively [50-53]. For example, generaly, with the increasing polarity of the solvent, an obvious decrease in the fluorescence emission of the charge transfer dyes and accompanied by a red-shift of the wavelength could be observed [54]. However, the increases of the solvent viscosity induced the fluorescence emission of molecular rotor dyes increases due to the restriction of intramolecular rotation [55].Thus, if the fluorescence bands of both a charge transfer dyeand an molecular rotor dye in an amine/alcohol mixture do not overlap, the fluorescence emission of the charge transfer dye will be dominant before reaction with CO2 while the fluorescence emission of the charge transfer dye will disappear along with the appearance of the fluorescence emission of the molecular rotor dye after reaction with CO2.
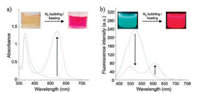
Based on above-mentioned mechanism, Kwak et al. developed a multidye-based fluorescent system for the detection of CO2 (Scheme 6) [56]. In this system, an equivalent molar mixture of 1, 8-diazabicyclo-[5.4.0]-undec-7-ene (DBU) and 1-propanol was selected as the CO2-sensitive smart solvent. Nile red and allyl-2-cyano-3-(1, 2, 3, 5, 6, 7-hexahydropyrido[3, 2, 1-ij]quinolin-9-yl)acrylate (7) were chosen as the charge transfer dye and molecular rotor dye respectively since Nile red displayed a very strong fluorescence emission with a maximum at 610 nm in the chosen smart solvent while 7 exhibited a weak fluorescence emissionwith a maximum at 489 nm in the same smart solvent.As shown in Fig. 10, in the absence of CO2, the smart solvent dissolved in Nile Red and 7 emited red light mainly due to Nile Red. However, after CO2 injection, the red-light emission of Nile red immediately disappears accompanied by the predomination of green-light emission from 7 owingtothe increase in polarity and viscosity, respectively. Moreover, it should be noted that the fluorescence color of the smart solvent system changed gradually with the increasing amount of CO2 injected (Fig. 11). For instance, the fluorescence color of the smart solvent system changed from red to pink, white, and then to light green with the CO2 molar ratio was 20%, 30%, and 40%, respectively. The results suggested that the determination of the amount of CO2 could be conducted by naked eye without the need for analytical equipment. Moreover, by simultaneously bubbling N2 gas and heating at 50 ℃, the green light-emission disappeared gradually along with the complete recovery of the initial red-light emission, which was attributed to the recovery of the initial viscosity and polarity values as a result of CO2 desorption (Fig. 12). Therefore, a fluorescent and colorimetric system for the detection of CO2 was achieved by combination of a charge transfer dye and a molecular rotor dye in smart solvent system.
|
Download:
|
| Scheme 6. Chemical structures and conceptual fluorescence response mechanism of the charge transfer dye (Nile red) and molecular rotor dye (7) to CO2 in the smart solvent system (equivalent molar DBU/1-propanol mixture). | |

|
Download:
|
| Fig. 10. (a) UV-vis absorption and (b) fluorescence emission (λex = 430 nm) spectra of Nile red and probe 7 coexisting in the DBU/1-propanol mixture (5.0 ×10-5 mol/L) before and after injection of an excess amount of CO2. Insets: Photographs of the solution under (a) ambient light and (b) UV light (λex > 365 nm). Dry ice was used as the CO2 source for experimental convenience. Reproduced with permission [56]. Copyright 2016, Elsevier Ltd. | |

|
Download:
|
| Fig. 11. Changes in (a) color, and (b) the UV-vis absorption and (c) fluorescence emission (λex = 430 nm) spectra of Nile red and probe 7 coexisting in the DBU/1-propanol mixture (5.0 ×10-5 mol/L) with the amount of injected CO2. Dry ice was used as the CO2 source for experimental convenience. Copied with permission [56]. Copyright 2016, Elsevier Ltd. | |
|
Download:
|
| Fig. 12. Changes in (a) UV-vis absorption and (b) fluorescence emission (λex = 430 nm) spectra of Nile red and probe 7 in the DBU/1-propanol mixture (5.0 ×10-5 mol/L) reacted with an excess amount of CO2 under simultaneous N2 gas bubbling and heating at 50 ℃. Copied with permission [56]. Copyright 2016, Elsevier Ltd. | |
6. Conclusions
During the past few years, we focused our research on the design and construction of novel small-molecule fluorescent probes and fluorescent materials [57-68]. Herein, in this review, we systematically summarized the development of the fluorescent probes for the detection of carbon dioxide. This review focuses on the design principles, recognition mechanisms, properties and functions of various kinds of small-molecule fluorescent probes for the detection of carbon dioxide, which presents a systematical summary of the development of the small-molecule fluorescent probes for the detection of carbon dioxide.
Although a great number of achievements have been received in this research field, in our opinion, two important aspects should be considered in the future development of new small-molecule fluorescent probes for carbon dioxide. Firstly, in order to develop and expand their biological applications, stable, biocompatible, water soluble, and near-infrared fluorescent probes should be designed and prepared. Secondly, most of the reported fluorescent probes can only be used to detect but not adsorb carbon dioxide. However, obviously, the adsorption of carbon dioxide is as important as detection of it. Thus, the preparation of functionalized fluorescent materials and fluorescent devices with the abilities to sense, separate, and adsorb carbon dioxide is particularly necessary. In a word, since carbon dioxide plays significantly important roles in many fields including chemical, environmental, clinical analysis, and agri-food industry, there is no doubt that such kinds of fluorescent probes will get much attention in the next decades.
AcknowledgmentsThanks to all excellent authors whose names appear in the references. We acknowledge the National Natural Science Foundation of China (Nos. 21871092, 21672070, 31570360), Shanghai Pujiang Program (No. 18PJD015), STCSM (Nos. 16XD1401000, 17XD14230000), and Shanghai Rising-Star Program (No. 16QB1403800) for the financial support.
| [1] |
D. Chen, H. Wang, L. Dong, et al., Biomaterials 103 (2016) 67-74. DOI:10.1016/j.biomaterials.2016.06.055 |
| [2] |
J. Sun, B. Ye, G. Xia, X. Zhao, H. Wang, Sens. Actuators B 233 (2016) 76-82. DOI:10.1016/j.snb.2016.04.052 |
| [3] |
L.H. Yang, H.M. Wang, ChemSusChem 7 (2014) 962-998. DOI:10.1002/cssc.201301131 |
| [4] |
C. Ziebart, C. Federsel, P. Anbarasan, et al., J. Am. Chem. Soc. 134 (2012) 20701-20704. DOI:10.1021/ja307924a |
| [5] |
C. Finn, S. Schnittger, L.J. Yellowlees, J.B. Love, Chem. Commun. 48 (2012) 1392-1399. DOI:10.1039/C1CC15393E |
| [6] |
O. Alduhaish, B. Li, H. Arman, et al., Chin. Chem. Lett. 28 (2017) 1653-1658. DOI:10.1016/j.cclet.2017.04.025 |
| [7] |
M.X. Wu, Y.W. Yang, Chin. Chem. Lett. 28 (2017) 1135-1143. DOI:10.1016/j.cclet.2017.03.026 |
| [8] |
F.Q. Meng, X.J. Feng, W.H. Wang, M. Bao, Chin. Chem. Lett. 28 (2017) 900-904. DOI:10.1016/j.cclet.2016.12.018 |
| [9] |
C.M. Kenific, J. Debnath, Trends Cell Biol. 25 (2015) 37-45. DOI:10.1016/j.tcb.2014.09.001 |
| [10] |
C. Descoins, M. Mathlouthi, M.L. Moual, J. Hennequin, J. Food Chem. 95 (2006) 541-553. DOI:10.1016/j.foodchem.2004.11.031 |
| [11] |
L.A. Blanchard, J.F. Brennecke, Ind. Eng. Chem. Res. 40 (2001) 287-292. DOI:10.1021/ie000710d |
| [12] |
O.S. Wolfbeis, L.J. Weis, M.J.P. Leiner, W.E. Ziegler, Anal. Chem. 60 (1988) 2028-2030. DOI:10.1021/ac00170a009 |
| [13] |
R.V. Geldern, M.E. Nowak, M. Zimmer, et al., Anal. Chem. 86 (2014) 12191-12198. DOI:10.1021/ac5031732 |
| [14] |
J.J. Gassensmith, J.Y. Kim, J.M. Holcroft, et al., J. Am. Chem. Soc. 136 (2014) 8277-8282. DOI:10.1021/ja5006465 |
| [15] |
T. Tian, X. Chen, H. Li, et al., Analyst 138 (2013) 991-994. DOI:10.1039/C2AN36401H |
| [16] |
L.Q. Xu, B. Zhang, M. Sun, et al., J. Mater. Chem. A 1 (2013) 1207-1212. DOI:10.1039/C2TA00482H |
| [17] |
R.N. Dansby-Sparks, J. Jin, S.J. Mechery, et al., Anal. Chem. 82 (2010) 593-600. DOI:10.1021/ac901890r |
| [18] |
M. Ishida, P. Kim, J. Choi, et al., Chem. Commun. 49 (2013) 6950-6952. DOI:10.1039/c3cc43938k |
| [19] |
W. Zheng, L.J. Chen, G. Yang, et al., J. Am. Chem. Soc. 138 (2016) 4927-4937. DOI:10.1021/jacs.6b01089 |
| [20] |
S. Pandey, S.N. Baker, S. Pandey, G.A. Baker, Chem. Commun. 48 (2012) 7043-7045. DOI:10.1039/c2cc32164e |
| [21] |
B. Jiang, J. Zhang, J.Q. Ma, et al., J. Am. Chem. Soc. 138 (2016) 738-741. DOI:10.1021/jacs.5b11409 |
| [22] |
B. Sun, M. Wang, Z. Lou, et al., J. Am. Chem. Soc. 137 (2015) 1556-1564. DOI:10.1021/ja511443p |
| [23] |
Y. Chen, T. Wei, Z. Zhang, et al., Chin. Chem. Lett. 28 (2017) 1957-1960. DOI:10.1016/j.cclet.2017.05.010 |
| [24] |
M. Lee, J.H. Moon, K.M.K. Swamy, et al., Sens. Actuators B 199 (2014) 369-376. DOI:10.1016/j.snb.2014.04.005 |
| [25] |
Z.H. Pan, G.G. Luo, J.W. Zhou, et al., Dalton Trans. 43 (2014) 8499-8507. DOI:10.1039/C4DT00395K |
| [26] |
R. Ali, S.S. Razi, R.C. Gupta, S.K. Dwivedi, A. Misra, New J. Chem. 40 (2016) 162-170. DOI:10.1039/C5NJ01920F |
| [27] |
S. Schutting, S.M. Borisov, I. Klimant, Anal. Chem. 85 (2013) 3271-3279. DOI:10.1021/ac303595v |
| [28] |
D.G. Khandare, H. Joshi, M. Banerjee, M.S. Majik, A. Chatterjee, Anal. Chem. 87 (2015) 10871-10877. DOI:10.1021/acs.analchem.5b02339 |
| [29] |
X. Zhang, S. Lee, Y. Liu, et al., Sci. Rep. 4 (2014) 4593-4600. |
| [30] |
Q. Xu, S. Lee, Y. Cho, et al., J. Am. Chem. Soc. 135 (2013) 17751-17754. DOI:10.1021/ja410557x |
| [31] |
X. Zhou, S. Lee, Z. Xu, J. Yoon, Chem. Rev. 115 (2015) 7944-8000. DOI:10.1021/cr500567r |
| [32] |
Y. Hong, J.W.Y. Lam, B.Z. Tang, Chem. Soc. Rev. 40 (2011) 5361-5388. DOI:10.1039/c1cs15113d |
| [33] |
J. Luo, Z. Xie, J.W.Y. Lam, et al., Chem. Commun. (2001) 1740-1741. |
| [34] |
L.J. Chen, Y.Y. Ren, N.W. Wu, et al., J. Am. Chem. Soc. 137 (2015) 11725-11735. DOI:10.1021/jacs.5b06565 |
| [35] |
Y. Liu, Y. Tang, N.N. Barashkov, et al., J. Am. Chem. Soc. 132 (2010) 13951-13953. DOI:10.1021/ja103947j |
| [36] |
H. Wang, D. Chen, Y. Zhang, et al., J. Mater. Chem. C 3 (2015) 7621-7626. DOI:10.1039/C5TC01280E |
| [37] |
S.N. Riduan, Y. Zhang, J.Y. Ying, Angew. Chem. Int. Ed. 48 (2009) 3322-3325. DOI:10.1002/anie.v48:18 |
| [38] |
Z. Guo, N.R. Song, J.H. Moon, et al., J. Am. Chem. Soc. 134 (2012) 17846-17849. DOI:10.1021/ja306891c |
| [39] |
M. Lee, S. Jo, D. Lee, Z. Xu, J. Yoon, Dyes Pigm. 120 (2015) 288-292. DOI:10.1016/j.dyepig.2015.04.029 |
| [40] |
R. Ali, S.S. Razi, M. Shahid, P. Srivastava, A. Misra, Spectrochim. Acta Part A 168 (2016) 21-28. DOI:10.1016/j.saa.2016.05.045 |
| [41] |
A.H. Liu, R. Ma, C. Song, et al., Angew. Chem. Int. Ed. 51 (2012) 11306-11310. DOI:10.1002/anie.201205362 |
| [42] |
Y. Zhang, S. Zhang, X. Lu, et al., Chem.-Eur. J. 15 (2009) 3003-3011. DOI:10.1002/chem.v15:12 |
| [43] |
W. Zheng, G. Yang, N. Shao, et al., J. Am. Chem. Soc. 139 (2017) 13811-13820. DOI:10.1021/jacs.7b07303 |
| [44] |
S. Kang, J. Kim, J.H. Park, et al., Dyes Pigm. 123 (2015) 125-131. DOI:10.1016/j.dyepig.2015.07.033 |
| [45] |
Z. Xu, J. Chen, L.L. Hua, et al., Chin. Chem. Lett. 28 (2017) 1935-1942. DOI:10.1016/j.cclet.2017.07.018 |
| [46] |
P. Zhang, Z.Q. Guo, C.X. Yan, W.H. Zhu, Chin. Chem. Lett. 28 (2017) 1952-1956. DOI:10.1016/j.cclet.2017.08.038 |
| [47] |
M.L. He, S. Wu, J. He, Z. Abliz, L. Xu, RSC Adv. 4 (2014) 2605-2608. DOI:10.1039/C3RA46500D |
| [48] |
C.B. Huang, J. Huang, L. Xu, RSC Adv. 5 (2015) 13307-13310. DOI:10.1039/C4RA08337G |
| [49] |
L. Phan, J.R. Andreatta, L.K. Horvey, et al., J. Org. Chem. 73 (2008) 127-132. DOI:10.1021/jo7017697 |
| [50] |
L. Song, X.D. Sun, Y. Ge, et al., Chin. Chem. Lett. 27 (2016) 1776-1780. DOI:10.1016/j.cclet.2016.05.007 |
| [51] |
Y. Yang, H. Wang, Y.L. Wei, et al., Chin. Chem. Lett. 28 (2017) 2023-2026. DOI:10.1016/j.cclet.2017.08.051 |
| [52] |
B. Jiang, L.J. Chen, Y. Zhang, et al., Chin. Chem. Lett. 27 (2016) 607-612. DOI:10.1016/j.cclet.2016.03.017 |
| [53] |
B. Li, Z. He, H. Zhou, H. Zhang, T. Cheng, Chin. Chem. Lett. 28 (2017) 1929-1934. DOI:10.1016/j.cclet.2017.08.055 |
| [54] |
T. Liu, X. Liu, D.R. Spring, et al., Sci. Rep. 4 (2014) 5418-5424. |
| [55] |
L. Wang, Y. Xiao, W. Tian, L. Deng, J. Am. Chem. Soc. 135 (2013) 2903-2906. DOI:10.1021/ja311688g |
| [56] |
Y.J. Jin, B.C. Moon, G. Kwak, Dyes Pigm. 132 (2016) 270-273. DOI:10.1016/j.dyepig.2016.05.003 |
| [57] |
C.B. Huang, L. Xu, J.L. Zhu, et al., J. Am. Chem. Soc. 139 (2017) 9459-9462. DOI:10.1021/jacs.7b04659 |
| [58] |
L.J. Chen, S. Chen, Y. Qin, et al., J. Am. Chem. Soc. 140 (2018) 5049-5052. DOI:10.1021/jacs.8b02386 |
| [59] |
H.I. Un, S. Wu, C.B. Huang, Z. Xu, L. Xu, Chem. Commun. 51 (2015) 3143-3146. DOI:10.1039/C4CC09488C |
| [60] |
Z. Xu, L. Xu, Chem. Commun. 52 (2016) 1094-1119. DOI:10.1039/C5CC09248E |
| [61] |
W.J. Fan, B. Sun, J. Ma, et al., Chem. Eur. J. 21 (2015) 12947-12959. DOI:10.1002/chem.201501282 |
| [62] |
J. Zhang, N.W. Wu, X.D. Xu, et al., RSC Adv. 4 (2014) 16047-16054. DOI:10.1039/C3RA46957C |
| [63] |
H.I. Un, C.B. Huang, J. Huang, et al., Chem. Asian J. 9 (2014) 3397-3402. DOI:10.1002/asia.v9.12 |
| [64] |
C.B. Huang, H.R. Li, Y. Luo, L. Xu, Dalton Trans. 43 (2014) 8102-8108. DOI:10.1039/c4dt00014e |
| [65] |
L. Wang, L.J. Chen, J.Q. Ma, et al., J. Organomet. Chem. 823 (2016) 1-7. DOI:10.1016/j.jorganchem.2016.09.001 |
| [66] |
C.B. Huang, L.J. Chen, J. Huang, L. Xu, RSC Adv. 4 (2014) 19538-19549. DOI:10.1039/c4ra02373k |
| [67] |
H.I. Un, C.B. Huang, C. Huang, et al., Org. Chem. Front. 1 (2014) 1083-1090. DOI:10.1039/C4QO00185K |
| [68] |
Y.Y. Ren, N.W. Wu, J. Huang, et al., Chem. Commun. 51 (2015) 15153-15156. DOI:10.1039/C5CC04789G |
 2018, Vol. 29
2018, Vol. 29 


