b NPU-QMUL Joint Research Institute of Advanced Materials and Structures(JRI-AMAS), Northwestern Polytechnical University, Xi'an 710072, China
Fluorescent metal nanoclusters (MNCs), usually consisting of several to approximately a hundred metal atoms, which bridge the gap between single metal atom and plasmonic metal nanoparticles (core sizes > 2 nm) [1], have attracted extensive attention over the past few decades. MNCs have size down to less than 2 nm, which is comparable to the Fermi wavelength of electrons [2], resulting in the breakup of the continuous density of states of the particles into discrete energy levels [1, 3]. MNCs exhibit particular optical, electronic and chemical properties, including strong photolumi-nescence, excellent photostability, good biocompatibility and sub-nanometer size. Such novel properties make MNCs ideal probes for many applications in biological imaging and diagnosis.
Near infrared (NIR) fluorescent MNCs are especially promising for bioimaging, because biological tissues show very low absorption and autofluorescence in the NIR spectrum window (650–900 nm wavelengths) [4, 5]. Also, NIR light can pass across several centimeters of heterogeneous living tissues [6]. Particularly, NIR-emitting MNC probes can alleviate several limitations of conventional NIR organic dyes and other nanoprobes like semiconductor quantum dots (QDs). Organic dyes show many drawbacks such as poor hydrophilicity and photostability, insufficient stability in biological systems and weak multiplexing capability [7]. Most reported QDs display high inherent cytotoxicity and self-aggregation inside live cells, which fatally limit their practical bio-applications [8].
Fluorescence lifetime imaging (FLIM) and two-photon imaging have been widely adopted in tissue and cell studies, and now have become powerful tools in early diseases detection and diagnosis as well as guiding the disease treatment [9, 10]. Fluorescent MNCs possess much longer lifetime than that of cellular autofluorescence and most organic dyes, making them attractive as markers for cellular FLIM applications. In contrast to fluorescence intensity imaging, lifetime-based imaging is independent of fluorophore concentration and laser excitation intensity [11]. Although one-photon fluorescence imaging techniques are featured with good spatial resolution and high sensitivity, they hardly obtain anatomical or three dimensional details of tumor tissues in vivo [12]. Compared to one-photon imaging, two-photon imaging is a powerful technique for enhanced tissue penetration depth (> 500 μm) with two NIR photon excitation, low tissue autofluorescence and self-absorption, as well as reduced photo-damage [10, 13, 14]. The relatively good biocompatibility and large two-photon absorption (TPA) cross section of MNCs make them ideal probes for two-photon imaging in biological system.
Besides fluorescence (FL) imaging, a number of other imaging techniques are also being used in the early-stage diagnosis of cancer, such as magnetic resonance imaging (MRI), X-ray computed tomography (CT), photoacoustic imaging (PAI), and single photon emission computed tomography (SPECT) [15, 16]. Each imaging modality has its own unique advantages along with intrinsic limitations [17]. For example, CT imaging can easily differentiate various tissue densities, and allow three-dimensional visual reconstructions of tissue; however, it suffers from poor sensitivity, especially in soft tissues with limited density differences [18, 19]. MR imaging is able to provide high-quality 3D information of soft tissues and possesses high spatial resolution, but has the disadvantage of relatively low sensitivity [20-23]. Therefore, the rational combination of different modalities, known as "multimodal imaging", is a powerful method that can provide more reliable and accurate detection of disease sites as it integrates the advantages of several imaging modalities and provides complementary information from each imaging modality. Consequently, it can provide more detailed anatomical or biological information about the target disease [15, 24].
In this review, we mainly focus on the latest progress in NIR-emitting MNC probes for biological imaging. Specifically, we summarize recent advances in the synthesis and applications of NIR-emitting MNCs (including Au, Ag, Cu and alloy NCs) as novel probes for bioimaging, including single modal imaging (fluorescence intensity-based imaging, FLIM, two-photon imaging) probes and the combination of NIRFL imaging with several other imaging techniques to form multimodal imaging (such as NIRFL/CT/MRI, NIRFL/PAI/MRI, NIRFL/SPECT) probes. In the final section, we will give a brief outlook on the challenges and opportunities for NIR-emitting MNCs in bioimaging applications.
2. Synthesis of NIR-emitting MNCsDuring the past decade, various methods have been developed to synthesize MNCs with the NIR-emitting property. Generally, there are two main strategies for preparing MNCs, named "bottom-up" and "top–down" synthetic routes [25]. For a "bottom-up" approach, template-assisted synthesis is usually adopted, where the corresponding metal precursors are reduced to atoms by reducing reagents and then the zero-valent metal atoms aggregate to form the nucleus of metal clusters under the protection of templates. Special templates such as thiols, biomolecules (including proteins, peptides and DNA), dendrimers and polymers have been used to direct the formation of NIR-emitting MNCs. In "top-down" method, ultrasmall size MNCs can be prepared from preformed large metal nanoparticles by a ligand-induced etching process. In the following, we will give a detailed overview for each synthetic strategy with various templates or capping ligands (representative examples summarized in Table 1).
|
|
Table 1 Summary of representative literatures on the synthesis of NIR-emitting MNCs. |
2.1. Thiols
Thiol-containing small molecules are the most commonly adopted stabilizers in MNC synthesis owing to the strong interaction between thiols and Au/Ag [26]. Among them, glutathione (GSH) is the most commonly adopted one. In a typical synthesis, GSH-stabilized AuNCs with a maximum emission at 780 nm could be obtained via NaBH4 reduction. These AuNCs display strong one- and two-photon emissions, good photo-stability and biocompatibility [27]. By employing GSH as reducing and protecting reagent simultaneously, Zhang et al. [28] successfully synthesized NIR-emitting GSH-AuNCs with a core size 2.5 nm at 90 ℃. Besides, water-soluble GSH-capped AuNCs were also obtained by using tetrabutylammonium borohydride (TBAB) as a mild reductant, and the yielded GSH-AuNCs showed excellent photoluminescence (PL) properties and low cytotoxicity [29]. Recently, Gao et al. [30] reported the intracellular biosynthesis of AgNCs by cancerous cells incubated with silver ions. AgNCs were spontaneously biosynthesized in situ by HeLa cancer cells treated with a specific silver salt derivative [Ag(GSH)]+ and exogenous GSH. Since GSH has two carboxylic functions, one may envision that [Ag(GSH)]+ consists of silver ions coordinated by the carboxylate moieties of GSH molecules, hence it is prone to give readily Ag(0) nuclei coordinated by GSH as soon as they penetrate into cancer cells. In stark contrast, treatment of L02 normal cells with [Ag(GSH)]+ did not lead to observable intracellular production of AgNCs.
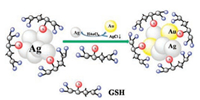
Using GSH as a scaffold and the novel sulfur-hydrazine hydrate complex (S–N2H4·H2O) as the S2- source, Wang et al. [31] developed a one-step approach to prepare water-soluble and biocompatibile fluorescent Ag2S NCs with tunable PL properties. By adjusting the amount of GSH and the ratio of Ag+ to S–N2H4·H2O, Ag2S NCs with different PL wavelengths and sizes were obtained. Subsequently, Wang et al. [32] used a facile galvanic replacement reaction to prepare Ag/Au alloy NCs. In the first step, the template (i.e., AgNCs) was prepared by using GSH as the stabilizing agent and N2H4·2H2O as the reducing agent. Then, when the AuCl4- ion and GSH were added to the aqueous solution of AgNCs, the galvanic replacement reaction occurred due to higher standard reduction potential of AuCl4-/Au pair (0.99 V vs. SHE) than that of Ag+/Ag pair (0.80 V vs. SHE) [33]. In this process, the Ag(0) changes to Ag+ and forms AgCl in the solution. On the other hand, the AuCl4- changes to Au(0) (Fig. 1). The as-prepared Ag/Au alloy NCs displayed NIR fluorescence centered at 716 nm and showed tunable luminescence from visible red (614 nm) to NIR (716 nm) by controlling the Ag/Au ratios.
|
Download:
|
| Fig. 1. The galvanic replacement reaction process of synthesizing NIR Ag/Au alloy NCs. Copied with permission [32]. Copyright 2014, Elsevier Publishing Group. | |
In addition to GSH, bidentate dihydrolipoic acid (DHLA) is another attractive ligand for MNC synthesis due to its strong binding affinity to metal atoms. Shang et al. [11] synthesized NIR-emitting DHLA–AuNCs with a one-pot strategy by simply reducing a mixture of lipoic acid (LA) and gold salt with NaBH4 in aqueous solution. The obtained AuNCs possess NIR emission and long fluorescence lifetime (> 100 ns), making them attractive as markers for cellular FLIM applications. Afterwards, the same group developed a microwave-assisted strategy for synthesizing DHLA-AuNCs [34]. Particularly, irradiation with microwaves during the synthesis enhanced the fluorescence quantum yield (QY) of AuNCs by about five-fold from ~0.6% to 2.9%, and it also shortened the reaction time from hours to several minutes. Moreover, by using microwave irradiation, the emission peak red shifts from 690 nm to 715 nm upon excitation at 580 nm. Later, via a slightly modified strategy, Nair et al. [35] reported the synthesis of NIR-emitting (Au)18(LA)14 NCs with a higher QY, 10%. Beside AuNCs, Ghosh et al. [36] reported the synthesis of brightly red fluorescent CuNCs in aqueous medium by using DHLA, in combination with biocompatible polymer poly(vinylpyrrolidone) (PVP) as stabilizers. The fluorescence of CuNCs was found to be sensitive to the pH of the medium, and the emission could be tuned reversibly according to the pH. The as-synthesized CuNCs at pH 4.5 emitted a red fluorescence at 650 nm. Besides GSH and DHLA, other thiols such as tiopronin and mercaptosuccinic acid (MSA) have also been used as stabilizers, which yielded AuNCs centered at 785 nm with QYs in the range of 3%–4% [37].
In addition to the above-mentioned one-pot strategy by direct reduction of metal ions in the presence of thiols, NIR-emitting MNCs can also be prepared by etching large metal nanoparticles with thiols. Au23(SG)18 NCs (SG denotes GSH) were obtained via the interfacial etching process using Au25SG18 NCs as the precursor. For interfacial etching, an interface was created by making an immiscible biphasic mixture of toluene containing octanethiol (OT) and an aqueous solution of Au25SG18. A highly fluorescent, water-soluble Au23(SG)18 cluster was obtained by etching at 25 ℃[38]. Red-emitting AgNCs were produced by an interfacial etching route using GSH as a ligand etchant from MSA-protected AgNPs. These AgNCs show high photostability over time and a high stability for a wide pH range [39]. Lin et al. [40] developed a strategy to synthesize DHLA-AuNCs based on precursor-induced AuNP etching in organic phase and ligand exchange with DHLA to transfer the particles into aqueous solution. Specifically, didode-cyldimethylammonium bromide (DDAB)-stabilized AuNPs (Au NPs@DDAB) are etched by the addition of Au precursors (HAuCl4 or AuCl3) to form smaller NCs (AuNCs@DDAB). Then hydrophobic AuNCs@DDAB become water-soluble upon ligand exchange with DHLA. Subsequently, the same group adopted a further 24 h thermal treatment at 70 ℃ to markedly increase the QY of AuNCs to nearly 7% [41].
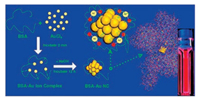
2.2. Proteins, peptides and DNA oligonucleotidesBiomacromolecules such as proteins and peptides have also been extensively utilized as templates for synthesizing NIR fluorescent MNCs. Compared with short peptides, large and complicated proteins possess abundant binding sites that can potentially bind and further reduce metal ions, thus offering better scaffolds for template-driven formation of small MNCs [42, 43]. Notably, bovine serum albumin (BSA) was first reported by Xie et al. as an excellent scaffold for AuNCs due to the strong force of Au–S bonding and the steric protection (Fig. 2), where NIR-emitting AuNCs with maximum emission wavelength at about 640 nm can be obtained by using BSA as both stabilizer and reductant [44]. Afterwards, Chen et al. [45] also employed the similar strategy to obtain NIR-emitting BSA-AuNCs under nitrogen conditions. Retnakumari et al. [46] and Guével et al. [47] successfully prepared highly fluorescent BSA-AuNCs by using ascorbic acid as a reducing agent, which showed an emission peak at around 670 nm. Inspired by these findings, researchers also tried many other proteins, such as transferrin-family proteins [48-50], trypsin [51], insulin [52] and ribonuclease A [53], as potential bioscaffolds for synthesizing NIR-emitting MNCs. For example, a one-pot approach was developed to prepare NIR-emitting transferrin (Tf)-templated CuNCs at room temperature via a biomineralization process, where ascorbic acid was used as the reductant. The as-prepared Tf-CuNCs exhibited intense NIR fluorescence with a QY about 6.2% [54]. Besides their well-known roles as capping agents, proteins such as BSA can also function as etching agents for synthesizing fluorescent AuNCs in a few cases. For example, Pradeep group [55] employed a core etching method to synthesize BSA-Au NCs from MSA-capped AuNPs.
|
Download:
|
| Fig. 2. Schematic of the formation of AuNCs in BSA solution under alkaline conditions. Copied with permission [44]. Copyright 2009, American Chemical Society. | |
Compared with proteins, synthetic peptides can resemble naturally occurring peptides and act as receptor binding or substrate recognition specific ligands. The integration of MNCs with synthetic peptides can also combine the distinct optical properties of MNCs with the biological functions of peptides [56]. In an elegant work, Gao group [57] developed a one-step biomineralization method to produce AuNCs by using a bifunctional CCYTAT peptide. The bifunctional peptide contains one domain with the ability to biomineralize and capture AuNCs and another domain that targets cell nuclei. The as-prepared AuNCs showed a maximum emission at 677 nm and possessed a high fluorescence QY of about 11%. Recently, by combining biomineralization and supramolecular self-assembly of motif-designed peptide constructs, researchers reported that the emission of peptide-AuNCs can be enhanced by nearly 70-fold, which largely increases their utility for biological applications [58].
The well-known interactions of metal cations with DNA have enabled the design and fabrication of various DNA-templated metal nanostructures [59]. Especially, the synthesis of small AgNCs usually adopt DNA oligonucleotides as stabilizers. In 2008, Dickson group [60] first reported the use of ssDNA to synthesize five distinct and essentially spectrally pure AgNC emitters with fluorescence tunable throughout the visible and NIR range. The NIR-emitting AgNCs formed in ammonium acetate buffered solutions at a pH range of 6.5–8, using oligonucleotides 5'-CCCTAACTCCCC-3' as the scaffolds. Notably, these AgNCs possessed a high QY up to 34%. Sharma et al. [61] also reported four different DNA sequences as AgNC templates with emission at different wavelengths. The resulting NIR-emitting AgNCs had QY greater than 50% and were very promising as biolabels. It has been shown in earlier reports that Ag+ has a higher binding affinity to cytosine bases than other bases [62-64]. Based on this principle, Antoku and coworkers [65, 66] reported NIR-emitting AgNCs creating in single stranded oligo-DNA consisting of 12 or 24 cytosine bases. By using more advanced DNA structures, such as G-quadruplex, as the template, Wang group [67] reported a novel approach to prepare fluorescent AgNCs made of 2–4 Ag atoms centered at 680 nm.
2.3. PolymersThere are also efforts on the synthesis of polymer-stabilized NIR-emitting MNCs based on their capability of sequestering metal ions from solutions. Moreover, the terminal groups on the polymer periphery are very useful for the further bioconjugation of MNCs. For instance, Huang et al. [68] prepared NIR-emitting AuNCs by a facile one-step NaBH4 reduction method using multidentate polymer, thioether-terminated poly(methacrylic acid) (PTMP-PMAA), as ligands. The resultant AuNCs showed an emission maximum near 660 nm with QY, 4.8%. In another report, fluorscent poly(ethylene glycol) (PEG)-AuNPs with an emission peak at 810 nm were created by thermally reducing HAuCl4 in the presence of thiolated PEG (PEG-SH) ligands with a molecular weight (MW) of 1 kDa (ca. 21 units) in aqueous solution [69]. Similarly, Wang et al. [70] reported a one-pot fabrication of thiol-terminated polyethyleneimine (SH-PEI) stabilized NIR-emitting AgNCs. SH-PEI not only acts as an excellent stabilizer for AgNCs, but also facilitates post-surface modification with functional biomolecules.
3. NIR-emitting MNCs as single modal bioimaging probesMNCs possess a series of attractive features including ultrasmall size, good biocompatibility, brightness and photostability, which render them as promising fluorescence probes for biological imaging. Especially, NIR-emitting MNCs have attracted more and more attention owing to their deeper tissue penetration and low autofluorescence background. Indeed, great progress has been achieved in recent years on employing NIR-emitting MNCs for biological imaging applications, as summarized in Table 2. In 2012, Shang et al. [34] demonstrated the utilization of DHLA-AuNCs for imaging intracellular Hg2+ in living HeLa cells, where they observed the intracellular fluorescence quenching effect upon addition of Hg2+ ions. Afterwards, they systematically varied the surface charge of human serum albumin (HSA) to examine the effect of Coulomb forces in modulating the biological interactions of AuNCs [72]. By utilizing confocal fluorescence microscopy to observe the uptake and localization of AuNCs in HeLa cells, they found distinct difference in the cellular uptake of AuNCs adsorbed with differently modified HSA (Fig. 3): nHSA (native HSA) suppressed cellular uptake, aHSA (HSA with more negative surface charges) showed negligible effect, and cHSA (HSA with more positive surface charges) enhanced cellular uptake. The results provide helpful information in designing NIR AuNCs aiming to highly efficient cell labeling applications.
|
|
Table 2 NIR-emitting MNCs as single modal fluorescence imaging probes. |

|
Download:
|
| Fig. 3. Three-dimensional fluorescence confocal images of HeLa cells upon incubation with AuNCs (2.5 μmol/L, green) for 2 h: (a) without proteins and with 2.5 μmol/L (b) nHSA, (c) aHSA and (d) cHSA. Cell membranes were stained with CellMask DeepRed (red). The data are shown as sections in the x-y plane (upper left), x-z plane (lower left) and y-z plane (right). Scale bar, 10 μm. Copied with permission [72]. Copyright 2014, Wiley Publishing Group. | |
Gao et al. [57] found that peptide–AuNCs with a bifunctional CCYTAT peptide could specifically target the nucleus of three different cell lines, including normal cells human gastric mucosa cells (GES-1), human embryonic lung fibroblast cells (MRC-5) and human cervical cancer cells (HeLa). Interestingly, they observed that the red emission from internalized AuNCs in the nucleus of HeLa cells is stronger than that in GES-1 and MRC-5 cells. Ai et al.[67] observed that G-quadruplex AS1411-templated AgNCs can specially bind nucleolin over-expressed cancer cells, thus can be directly employed in bioimaging HeLa cells. Besides, Guével et al.[39] employed GSH-AgNCs as optical probes for NIR fluorescence imaging of epithelial lung cancer A549 cells. Confocal images showed that AgNCs were taken up in the cytoplasm and more specifically in the vesicles of A549 cells, but were absent in the nucleus. In contrast, Wang and coworkers [31, 32] observed that GSH-capped Ag2S NCs and Ag/Au alloy NCs were not only distributed in cytoplasm but mostly in the cellular nucleus of MC3T3-EI cells and CAL-27 cells. These differences in the intracellular localization of MNCs upon the internalization suggest that not only the surface ligands but also the cell types can influence their intracellular fate.
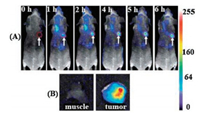
In addition to in vitro cellular imaging, a large number of reports have also focused on tumor imaging in vivo currently. For example, Wu et al. [71] reported the first example of tumor imaging with BSA-AuNCs. Fluorescence images from these NIR-emitting AuNCs exhibited high contrast and could be easily distinguished from the background. Furthermore, in vivo tumor targeting and ex vivo imaging studies showed that these ultrasmall AuNCs were able to be highly accumulated in the tumor areas (Fig. 4), thanks to the enhanced permeability and retention (EPR) effects. Sun et al. [49] achieved ferritin receptor-mediated targeting and bioimaging with far-red emitting paired AuNCs. Their study demonstrated that farred AuNCs could act as an excellent probe for targeting ferritin receptor-overexpressed human Caco-2 cells and whole female nude mice body imaging with specific targeting to the kidney. In addition, renal-clearable NIR-emitting GSH-AuNCs and PEG-AuNCs have been reported for in vivo NIR tumor targeting of MCF-7 tumor-bearing mice [28, 69]. These NIR-emitting AuNCs not only exhibited efficient renal clearance and low reticuloendothelial system (RES) accumulation, but also showed a much longer tumor retention time and faster normal tissue clearance, which is likely due to the well-known EPR effect.
|
Download:
|
| Fig. 4. (A) Fluorescence images of mice bearing an MDA-MB-45 tumor. Strong signal from AuNCs was observed in the tumor (marked by the red circle). The arrowheads indicated the tumor. (B) Ex vivo fluorescence image of the tumor tissue and the muscle tissue around the tumor from the mice used in A. Reproduced with permission [71]. Copyright 2010, Royal Society of Chemistry. | |
Furthermore, Gao et al. [30] reported a novel strategy of in situ self-imaging cancer cells and tumors through bio-synthesized NIR-emitting AgNCs, while this did not occur in normal cells and tissues. Interestingly, the spontaneous self-generation of AgNCs by xenografted tumors over several (7–14) days led to a significant decrease in tumor sizes. Meanwhile, the relevant body weight had little or almost no change for the mice after treated by [Ag(GSH)]+. However, for the non-treated group, the mice body weight dramatically decreased, accompanied with significant increase of the tumor size. Besides, Liu et al. [52] successfully synthesized fluorescent human insulin–Au nanodots (NDs) for in vivo imaging of insulin metabolism. Investigations on mice ear and ex vivo assays on human fat tissues showed that cells with rich insulin receptors had higher uptake of administrated insulin. Interestingly, the insulin–AuNDs could even permeate into lipid droplets (LDs) of adipocytes, and the epicardial adipocytes of patients with diabetes and coronary artery disease showed elevated adjacent/LD concentration contrast. As a result, human insulin–AuNDs provided a new approach to explore subcellular insulin metabolism in model animals or patients with metabolic or cardiovascular diseases.
For the purpose of targeted imaging of cancer cells and tumors, fluorescent MNCs are modified with specific recognition units such as folic acid (FA) and streptavidin. For example, targeted imaging of folate receptor (FR) positive oral carcinoma KB cells using FA conjugated BSA-AuNCs has been reported [46, 55]. Tumor-targeting and specific affinity of FA-conjugated AuNCs for FR overexpressed tumors facilitated the accumulation of AuNCs in the tumor site, which enhanced the NIR fluorescence signal in the tumor site, enabling in vivo targeted imaging of tumors with high specificity and also the subsequent tumor therapy [35, 45, 51]. Recently, Wang et al. [70] reported the conjugation of PEI-AgNCs with FA for both in vitro and in vivo targeted imaging. Their results indicated that the clearance rate of FA-conjugated AgNCs in the tumor bearing mice was much slower than that in the normal mice because the high affinity of FA to target tumors inhibited FA-AgNCs from being metabolized. Similar to FA-conjugated AgNCs, streptavidin-conjugated AuNCs have been reported to specifically label endogenous biotin within human hepatoma cells (HepG2) using the specific interactions between streptavidin and biotin [38, 40].
Apart from FA and streptavidin, other functionalized molecules have also been used to conjugate with MNCs. For instance, Kong et al. [53] developed a multifunctional nanoprobe for simultaneous targeting and imaging of human colon carcinoma Caco-2 cells by conjugating vitamin B12 to the ribonuclease A–stabilized AuNCs. Chen et al. [73] fabricated a fluorescent nanoprobe capable of specifically targeting carcinoma cells and tumors by coupling methionine (Met) and an NIR organic fluorescent dye MPA to BSA-AuNCs (Au-Met-MPA). Low toxicity of this probe as well as its tumor targeting capability was demonstrated by both in vitro and in vivo studies. The cancer cell imaging results showed that Au-Met-MPA exhibited a relatively higher accumulation in MCF-7 tumor cells (Met receptor positive) in comparison with L02 human normal cells. The in vivo tumor imaging results showed that Au-Met-MPA exhibited a relatively higher tumor-targeting distribution in LAT1 and LAT2-positive tumor-bearing mice models (MDA-MB-231) when compared with those of relatively low LAT1 and LAT2 expressing tumor models (A549). Cui et al. [74] synthesized well-defined AuNC nanoassembly by the self-assembly of reduced AuNCs using GSH as linkers. The as-prepared nanoassembly displayed highly effective cellular uptake and precise tumor targeting for NIRFL imaging in vivo compared to that of individual AuNCs. Wang et al. [48] reported the fabrication of Tf-AuNCs/graphene oxide (GO) nanocomposite (Tf-AuNCs/GO) for turn-on NIR fluorescence bioimaging of transferrin receptor (TfR) over-expressed HeLa cells and HeLa tumor-bearing mice. GO could markedly quench the fluorescence of Tf-AuNCs due to the fluorescence resonance energy transfer (FRET), which can be effectively restored in the presence of TfR, due to the highly specific interactions between Tf and TfR that can competitively bind and desorb TfR from the Tf-AuNCs/GO composite.
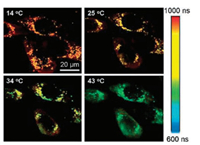
FLIM is a powerful technique for cell imaging that takes advantages of longer fluorescence lifetime of MNCs than that of autofluorescence from cellular organelles. For example, the average fluorescence lifetime of DHLA–AuNCs (500–800 ns) is two orders of magnitude longer than that of cellular autofluorescence (1.5–4 ns), so that they can easily be imaged by using lifetime gating. Upon FLIM imaging, the researchers observed that AuNCs located near the cell membrane displayed longer lifetimes than those internalized inside the cells [11], indicating that FLIM imaging not only reveals the cellular uptake of AuNCs but also provides information on their different local environment. In a later work, based on the fact that the fluorescence intensity as well as the lifetime of DHLA-AuNCs is highly dependent on the temperature, Shang et al. [75] demonstrated the utilization of AuNC-based FLIM imaging for temperature sensing in live cells. As shown in Fig. 5, these images provide clear evidences that the long fluorescence lifetime components in the range of 600–1000 ns arise from internalized AuNCs rather than cellular autofluorescence. With increasing the temperature, the fluorescence lifetime decreased markedly from 970 ns at 14 ℃ to 670 ns at 43 ℃, suggesting the potential of AuNC-based system for thermal sensing at the subcellular level via FLIM. In another report, Zhang et al. [37] demonstrated FLIM-based cellular imaging by using MSA- and tiopronin-capped AuNCs and further covalently bound PEG moieties to improve their capability of staining HeLa cells. Particularly, they observed that these PEGlyated AuNCs widely distribute throughout the cells and especially accumulate in the areas close to the cell nucleus.
|
Download:
|
| Fig. 5. Typical FLIM images of HeLa cells with internalized AuNCs at four different temperatures. Copied with permission [75]. Copyright 2013, Wiley Publishing Group. | |
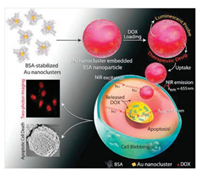
Two-photon imaging is advantageous for studying biological samples because of its ability of imaging depth inside tissues and low phototoxicity of NIR light. The outstanding TPA cross sections of MNCs make them good candidates for application in two-photon cellular imaging. Polavarapu et al. [27] investigated the two-photon excitation fluorescence imaging of SH-SY5Y human neuroblastoma cells incubated with GSH-AuNCs under excitation of femtosecond laser pulses at 800 nm. The two-photon imaging and Z-stack sectioning results clearly confirmed that AuNCs were internalized inside the cells, and no auto-fluorescence from cells was observed. Khandelia et al. [76] reported the use of hydrophilic anticancer drug doxorubicin (DOX) loaded BSA-AuNCs for imaging the human cervical cancer HeLa cells by two-photon excitation at 730 nm. Their results demonstrated that DOX-loaded AuNCs not only helped in tracking the delivery but also released drugs to the cancer cells, leading to apoptotic cell death (Fig. 6). In a recent work, Gu et al. [77] prepared RGD conjugated BSA-AuNC nano-capsules for two-photon fluorescence imaging of U87-MG cancer cells. Under the two-photon excitation of 820 nm, U87-MG cells treated with RGD-labeled AuNC nano-capsules exhibited a strong red luminescence centered at 650 nm. In addition, the Z-stack sectioning of two-photon images revealed that hybrid nano-capsules were mainly resided in the cytoplasm nearby the nucleus.
|
Download:
|
| Fig. 6. A schematic illustration of the formation of DOX-loaded AuNC-embedded BSA nanoparticles, followed by uptake and release of DOX inside HeLa cells, leading to apoptotic cell death, as visualized by two-photon imaging. Copied with permission [76]. Copyright 2015, Wiley Publishing Group. | |
4. NIR-emitting Au NCs as multimodal bioimaging probes
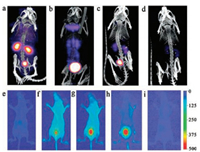
At present, multimodal imaging probes based on fluorescent AuNCs for tumor imaging have also attracted plenty of attention (see the summary in Table 3). In an early work, Zhou et al. [78] reported multimodal imaging of NIR-emitting radioactive GSH-AuNPs, which were incorporated with a gold radioisotope 198Au. The 198Au in GSH-[198Au] AuNPs not only helps to quantify the pharmacokinetics of these NIR-emitting AuNPs rapidly, but also allows their utility for in vivo SPECT imaging by emitting gamma rays. Thus these NIR-emitting radioactive AuNPs can serve as dualmodality imaging probes with both SPECT and FL imaging capabilities (Fig. 7). Chen et al. [79] recently fabricated a dual-modality FL/CT iodinated BSA-AuNCs for early accurate diagnosis of thyroid cancer. They accomplished in vivo FL and CT imaging via an orthotopic human thyroid cancer patient tissue derived xenograft (PDX) mouse model. Adopting the similar FL and CT dual-modal imaging techniques, insulin–AuNCs were used to distinguish the differentiated C2C12 myoblasts from undifferentiated ones [80]. Also, FA-conjugated GSH-AuNCs and lysozyme-AuNCs have been used for in vivo targeted dual-modal FL/CT imaging of MGC-803 tumour-bearing mice and HeLa tumor-bearing nude mice, respectively [29, 81].
|
|
Table 3 NIR-emitting AuNCs as multimodal bioimaging probes. |
|
Download:
|
| Fig. 7. Representative SPECT images (top row) of balb/c mice injected with GSH-[198Au] AuNPs. (a) 10 min, (b) 1 h, (c) 4 h, and (d) 24 h p.i. In vivo FL imaging (bottom row) of a live mouse. (e) pre-injection, and (f) 5 min, (g) 20 min, (h) 1 h, (i) 24 h after Ⅳ injection of GSH-[198Au] AuNPs. Reproduced with permission [78]. Copyright 2012, Wiley Publishing Group. | |
Furthermore, NIRFL and MR dual-modal imaging have been reported through coupling AuNCs with magnetic agents such as Gd2O3 and Fe3O4 NPs. For example, Sun et al. [82] employed Gd2O3 functionalized BSA-AuNCs as probes for dual-modal NIRFL and MR blood pool imaging in vivo. By further bioconjugation of BSA-Gd2O3/AuNCs with arginine–glycine–aspartic acid peptide (RGD), they can be used for in vivo targeted tumor imaging of U87-MG tumor-bearing mice. Liang et al. [83] proposed a simple strategy to construct Gd3+-functionalized AuNCs for dual-model NIRFL/MR imaging by using a cyclodecapeptide as the template. In particular, the ability of Gd-AuNCs to freely circulate in the blood pool without obvious uptake by the RES (i.e., the liver and spleen), along with the fact that the materials can be removed from the body through renal clearance, is very attractive for high-resolution blood pool imaging. Recently, Wang et al. [84] demonstrated a facile strategy of fabricating GSH-AuNC probes decorated with magnetic Fe3O4 NPs for bimodal NIRFL/MR cell imaging. Alternatively, dual-modal bioimaging probes can be fabricated by the conjugation of biotinylated NIR fluorescent BSA-AuNCs to streptavidin functionalized Fe3O4 NPs [85].
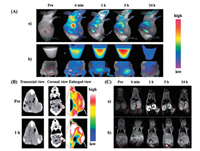
In addition to the NIRFL and MRI contrasts offered by the probe, the green fluorescence of the endoperoxide triggered by 1O2 can provide a third modality for live cell imaging [85]. With the co-existence of GSH-AuNCs and Gd3+ ions, the nanoprobes can act as a multifunctional nanoplatform for triple-modal NIRFL/CT/MR imaging of A549 cancer cells and xenografted A549 tumor models [86]. Similarly, Hu et al. [87] prepared Au–Gd NC hybrids by using albumin as the stabilizer, which were suitable for in vivo triple-modal NIRFL/CT/MRI imaging of MCF-7 tumor-bearing mice (Fig. 8). Upon intravenously injected, the hybrid NCs were effectively accumulated in tumor tissues and quickly cleared by renal excretion, indicating their capacity of tumor targeting and low body residues. Xu et al. [88] synthesized a tri-modal imaging agent, composed of AuNC-Gd2O3 integrated nanoprobe (denoted as AuGds) using BSA as the template via a bio-mineralization approach. After being chemically modified with FA, the FA-AuGds could specifically target FRs on KB tumor cells, and permitted in vivo NIRFL, MR, and CT imaging of xenografted KB tumor-bearing mice. Besides, Hembury et al. [89] synthesized highly mono-dispersed SiO2/AuNCs by nucleating gold within hollow meso-porous silica particles in a one-phase synthetic route. These SiO2/AuNCs possessed stable NIR fluorescence and paramagnetism, thus it could be used as a promising probe for in vivo NIRFL/PAI/MR imaging of Colorectal carcinoma tumor (LS174T)-bearing mice.
|
Download:
|
| Fig. 8. (A) In vivo FL imaging of MCF-7 tumor bearing mice after the tail-vein injection of hybrid NCs. Inset image is the FL reflectance images of urine. (B) In vivo CT images of MCF-7 tumor-bearing mice injected with the hybrid Au–Gd NCs. The arrow and red dotted circle indicate the tumor site. (C) (a and b) In vivo MRI images of MCF-7 tumor-bearing mice injected with the hybrid Au–Gd NCs. The arrow and red dotted circle indicate the tumor (a) and bladder (b) sites, respectively. Copied with permission [87]. Copyright 2013, Royal Society of Chemistry. | |
5. Conclusions
In this review, we have systematically summarized recent advances in the synthesis strategies and bioimaging applications of NIR-emitting MNCs. In the past few years, NIR-emitting MNCs have been largely explored for bioimaging due to their utrasmall size, good biocompatibility, and especially their long fluorescence lifetime, deeper tissue penetration and low autofluorescence background. Although a large number of researches have been reported about NIR-emitting MNCs currently, there are still a lot of rooms to further improve.
First of all, most of the currently reported MNCs possess a relatively low QY (usually less than 10%) in comparison to semiconductor QDs and many organic dyes. In addition, MNCs often show size heterogeneity in the crude product. Therefore, researchers still need to make greater effort to explore more efficient synthesis routes for size-controllable fluorescent MNCs with relatively high QY and high purity [91, 92]. A clear understanding on the photoluminescence mechanism of these new nano-fluorophores will be particularly helpful, which can further advance the development of more robust synthetic strategies of NIR-emitting MNCs. Second, recent bioimaging studies mainly concentrate on NIR fluorescent AuNCs. Considering gold is relatively expensive compared to other metals, it would be attractive to explore the synthesis and bioimaging applications of other NIR-emitting MNCs or alloy NCs without compromising their essential features. Third, despite many reports on the bioimaging applications of fluorescent MNCs, relatively little is known about the interaction of these ultrasmall clusters with the biological environment [93]. The in vitro and in vivo behavior of MNCs is related to the safe as well as efficient use of MNCs in biological systems. Thus, further study to understand the mechanism of cellular and intravital uptake of MNCs and long-term effect after entering into bio-systems would be appreciated.
Overall, although significant progress has been achieved in the field of developing NIR-emitting MNCs for bioimaging, many challenges still remain to face and resolve in the future. With continuing development, we believe that more robust NIR fluorescent MNCs will be available, which will then further advance imaging-based applications of these novel nanoprobes in medical diagnose and therapy researches.
AcknowledgmentsFinancial supports from the National 1000 Young Talent Program, National Natural Science Foundation of China (NSFC, No. 21705129) and Program of Introducing Talents of Discipline to Universities (No. B08040) are acknowledged.
| [1] |
I. Díez, R.H.A. Ras, Few-Atom Silver Clusters as Fluorescent Reporters, Advanced Fluorescence Reporters in Chemistry and Biology Ⅱ, Springer, 2010, pp. 307-332.
|
| [2] |
J. Zheng, P.R. Nicovich, R.M. Dickson, Annu. Rev. Phys. Chem. 58 (2007) 409-431. DOI:10.1146/annurev.physchem.58.032806.104546 |
| [3] |
I. Diez, R.H. Ras, Nanoscale 3 (2011) 1963-1970. DOI:10.1039/c1nr00006c |
| [4] |
X. He, K. Wang, Z. Cheng, WIREs Nanomed. Nanobiotechnol. 2 (2010) 349-366. DOI:10.1002/wnan.85 |
| [5] |
F. Song, R. Liang, J. Deng, et al., Chin. Chem. Lett. 28 (2017) 1997-2000. DOI:10.1016/j.cclet.2017.08.023 |
| [6] |
H. Park, K.B. Crozier, Sci. Rep. 3 (2013) 2460. DOI:10.1038/srep02460 |
| [7] |
X. He, J. Gao, S.S. Gambhir, et al., Trends Mol. Med. 16 (2010) 574-583. DOI:10.1016/j.molmed.2010.08.006 |
| [8] |
J. Weng, J. Ren, Curr. Med. Chem. 13 (2006) 897-909. DOI:10.2174/092986706776361076 |
| [9] |
M.Y. Berezin, S. Achilefu, Chem. Rev. 110 (2010) 2641-2684. DOI:10.1021/cr900343z |
| [10] |
F. Helmchen, W. Denk, Nat. Methods 2 (2005) 932-940. DOI:10.1038/nmeth818 |
| [11] |
L. Shang, N. Azadfar, F. Stockmar, et al., Small 7 (2011) 2614-2620. DOI:10.1002/smll.v7.18 |
| [12] |
L. Yuan, W. Lin, K. Zheng, et al., Chem. Soc. Rev. 42 (2013) 622-661. DOI:10.1039/C2CS35313J |
| [13] |
H.M. Kim, C. Jung, B.R. Kim, et al., Angew. Chem. Int. Ed. 46 (2007) 3460-3463. |
| [14] |
W.R. Zipfel, R.M. Williams, W.W. Webb, Nat. Biotechnol. 21 (2003) 1369-1377. DOI:10.1038/nbt899 |
| [15] |
D.E. Lee, H. Koo, I.C. Sun, et al., Chem. Soc. Rev. 41 (2012) 2656-2672. DOI:10.1039/C2CS15261D |
| [16] |
D.Z. Li, H.D. Chen, F. Bi, et al., Chin. J. Anal. Chem. 44 (2016) 1609-1618. DOI:10.1016/S1872-2040(16)60966-0 |
| [17] |
J.K. Willmann, N. van Bruggen, L.M. Dinkelborg, et al., Nat. Rev. Drug Discov. 7 (2008) 591-607. DOI:10.1038/nrd2290 |
| [18] |
B. Zhou, L. Zheng, C. Peng, et al., ACS Appl. Mater. Interfaces 6 (2014) 17190-17199. DOI:10.1021/am505006z |
| [19] |
C. Peng, J. Qin, B. Zhou, et al., Polym. Chem. 4 (2013) 4412-4424. DOI:10.1039/c3py00521f |
| [20] |
J. Zhou, Z. Lu, G. Shan, et al., Biomaterials 35 (2014) 368-377. DOI:10.1016/j.biomaterials.2013.09.088 |
| [21] |
J. Kim, Y. Piao, T. Hyeon, Chem. Soc. Rev. 38 (2009) 372-390. DOI:10.1039/B709883A |
| [22] |
M. Tsotsalas, M. Busby, E. Gianolio, et al., Chem. Mater. 20 (2008) 5888-5893. DOI:10.1021/cm8006183 |
| [23] |
J.H. Lee, Y.M. Huh, Y.W. Jun, et al., Nat. Med. 13 (2007) 95-99. DOI:10.1038/nm1467 |
| [24] |
A. Louie, Chem. Rev. 110 (2010) 3146-3195. DOI:10.1021/cr9003538 |
| [25] |
Y. Lu, W. Chen, Chem. Soc. Rev. 41 (2012) 3594-3623. DOI:10.1039/c2cs15325d |
| [26] |
R. Jin, Nanoscale 2 (2010) 343-362. DOI:10.1039/B9NR00160C |
| [27] |
L. Polavarapu, M. Manna, Q.H. Xu, Nanoscale 3 (2011) 429-434. DOI:10.1039/C0NR00458H |
| [28] |
J. Liu, M. Yu, C. Zhou, et al., J. Am. Chem. Soc. 135 (2013) 4978-4981. DOI:10.1021/ja401612x |
| [29] |
C. Zhang, Z. Zhou, Q. Qian, et al., J. Mater. Chem. B 1 (2013) 5045-5053. |
| [30] |
S. Gao, D. Chen, Q. Li, et al., Sci. Rep. 4 (2014) 4384. |
| [31] |
C. Wang, Y. Wang, L. Xu, et al., Small 8 (2012) 3137-3142. DOI:10.1002/smll.v8.20 |
| [32] |
C. Wang, L. Xu, X. Xu, et al., J. Colloid Interface Sci. 416 (2014) 274-279. DOI:10.1016/j.jcis.2013.11.011 |
| [33] |
Y. Sun, Y. Xia, J. Am. Chem. Soc. 126 (2004) 3892-3901. DOI:10.1021/ja039734c |
| [34] |
L. Shang, L. Yang, F. Stockmar, et al., Nanoscale 4 (2012) 4155-4160. DOI:10.1039/c2nr30219e |
| [35] |
L.V. Nair, S.S. Nazeer, R.S. Jayasree, et al., ACS Nano 9 (2015) 5825-5832. DOI:10.1021/acsnano.5b00406 |
| [36] |
R. Ghosh, U. Goswami, S.S. Ghosh, et al., ACS Appl. Mater. Interfaces 7 (2015) 209-222. DOI:10.1021/am505799q |
| [37] |
J. Zhang, Y. Fu, C.V. Conroy, et al., J. Phys. Chem. C 116 (2012) 26561-26569. DOI:10.1021/jp306036y |
| [38] |
M.A. Muhammed, P.K. Verma, S.K. Pal, et al., Chem.-Eur. J. 15 (2009) 10110-10120. DOI:10.1002/chem.v15:39 |
| [39] |
X. Le Guével, C. Spies, N. Daum, et al., Nano Res. 5 (2012) 379-387. DOI:10.1007/s12274-012-0218-1 |
| [40] |
C.A.J. Lin, T.Y. Yang, C.H. Lee, et al., ACS Nano 3 (2009) 395-401. DOI:10.1021/nn800632j |
| [41] |
H.H. Wang, C.A.J. Lin, C.H. Lee, et al., ACS Nano 5 (2011) 4337-4344. DOI:10.1021/nn102752a |
| [42] |
L. Shang, S. Dong, G.U. Nienhaus, Nano Today 6 (2011) 401-418. DOI:10.1016/j.nantod.2011.06.004 |
| [43] |
L. Shang, G.U. Nienhaus, Nat. Chem. 7 (2015) 769-770. DOI:10.1038/nchem.2357 |
| [44] |
J. Xie, Y. Zheng, J.Y. Ying, J. Am. Chem. Soc. 131 (2009) 888-889. DOI:10.1021/ja806804u |
| [45] |
H. Chen, S. Li, B. Li, et al., Nanoscale 4 (2012) 6050-6064. DOI:10.1039/c2nr31616a |
| [46] |
A. Retnakumari, S. Setua, D. Menon, et al., Nanotechnology 21 (2010) 055103. DOI:10.1088/0957-4484/21/5/055103 |
| [47] |
X. Le Guével, B. Hötzer, G. Jung, et al., J. Mater. Chem. 21 (2011) 2974-2981. DOI:10.1039/c0jm02660c |
| [48] |
Y. Wang, J.T. Chen, X.P. Yan, Anal. Chem. 85 (2013) 2529-2535. DOI:10.1021/ac303747t |
| [49] |
C. Sun, H. Yang, Y. Yuan, et al., J. Am. Chem. Soc. 133 (2011) 8617-8624. DOI:10.1021/ja200746p |
| [50] |
X. Le Guevel, N. Daum, M. Schneider, Nanotechnology 22 (2011) 275103. DOI:10.1088/0957-4484/22/27/275103 |
| [51] |
J.M. Liu, J.T. Chen, X.P. Yan, Anal. Chem. 85 (2013) 3238-3245. DOI:10.1021/ac303603f |
| [52] |
C.L. Liu, T.M. Liu, T.Y. Hsieh, et al., Small 9 (2013) 2103-2110. DOI:10.1002/smll.v9.12 |
| [53] |
Y. Kong, J. Chen, F. Gao, et al., Nanoscale 5 (2013) 1009-1017. DOI:10.1039/C2NR32760K |
| [54] |
T. Zhao, X.W. He, W.Y. Li, et al., J. Mater. Chem. B 3 (2015) 2388-2394. DOI:10.1039/C4TB02130D |
| [55] |
M.A. Habeeb Muhammed, P.K. Verma, S.K. Pal, et al., Chem.-Eur. J. 16 (2010) 10103-10112. DOI:10.1002/chem.201000841 |
| [56] |
Q. Yuan, Y. Wang, L. Zhao, et al., Nanoscale 8 (2016) 12095-12104. DOI:10.1039/C6NR02750D |
| [57] |
Y. Wang, Y. Cui, Y. Zhao, et al., Chem. Commun. 48 (2012) 871-873. DOI:10.1039/C1CC15926G |
| [58] |
W. Zhang, D. Lin, H. Wang, et al., Bioconjugate Chem. 28 (2017) 2224-2229. DOI:10.1021/acs.bioconjchem.7b00312 |
| [59] |
S. Pitchiaya, Y. Krishnan, Chem. Soc. Rev. 35 (2006) 1111-1121. DOI:10.1039/b602886c |
| [60] |
C.I. Richards, S. Choi, J.C. Hsiang, et al., J. Am. Chem. Soc. 130 (2008) 5038-5039. DOI:10.1021/ja8005644 |
| [61] |
J. Sharma, H.C. Yeh, H. Yoo, et al., Chem. Commun. 46 (2010) 3280-3282. DOI:10.1039/b927268b |
| [62] |
S. Shukla, M. Sastry, Nanoscale 1 (2009) 122-127. DOI:10.1039/b9nr00004f |
| [63] |
V. Soto-Verdugo, H. Metiu, E. Gwinn, J. Chem. Phys. 132 (2010) 195102. DOI:10.1063/1.3419930 |
| [64] |
D. Schultz, E. Gwinn, Chem. Commun. 47 (2011) 4715-4717. DOI:10.1039/c0cc05061j |
| [65] |
T. Vosch, Y. Antoku, J.C. Hsiang, et al., Proc. Natl. Acad. Sci. U. S. A. 104 (2007) 12616-12621. DOI:10.1073/pnas.0610677104 |
| [66] |
Y. Antoku, J. Hotta, H. Mizuno, et al., Photochem. Photobiol. Sci. 9 (2010) 716-721. DOI:10.1039/c0pp00015a |
| [67] |
J. Ai, W. Guo, B. Li, et al., Talanta 88 (2012) 450-455. DOI:10.1016/j.talanta.2011.10.057 |
| [68] |
X. Huang, Y. Luo, Z. Li, et al., J. Phys. Chem. C 115 (2011) 16753-16763. DOI:10.1021/jp202612p |
| [69] |
J. Liu, M. Yu, X. Ning, et al., Angew. Chem. Int. Ed. 52 (2013) 12572-12576. DOI:10.1002/anie.201304465 |
| [70] |
Y. Wang, C. Dai, X.P. Yan, Chem. Commun. 50 (2014) 14341-14344. DOI:10.1039/C4CC06329E |
| [71] |
X. Wu, X. He, K. Wang, et al., Nanoscale 2 (2010) 2244-2249. DOI:10.1039/c0nr00359j |
| [72] |
L. Shang, L. Yang, J. Seiter, et al., Adv. Mater. Interfaces 1 (2014) 1300079. DOI:10.1002/admi.201300079 |
| [73] |
H. Chen, B. Li, X. Ren, et al., Biomaterials 33 (2012) 8461-8476. DOI:10.1016/j.biomaterials.2012.08.034 |
| [74] |
H.D. Cui, D.H. Hu, J.N. Zhang, et al., Chin. Chem. Lett. 28 (2017) 1391-1398. DOI:10.1016/j.cclet.2016.12.038 |
| [75] |
L. Shang, F. Stockmar, N. Azadfar, et al., Angew. Chem. Int. Ed. 52 (2013) 11154-11157. DOI:10.1002/anie.201306366 |
| [76] |
R. Khandelia, S. Bhandari, U.N. Pan, et al., Small 11 (2015) 4075-4081. DOI:10.1002/smll.v11.33 |
| [77] |
W. Gu, Q. Zhang, T. Zhang, et al., J. Mater. Chem. B 4 (2016) 910-919. DOI:10.1039/C5TB01619C |
| [78] |
C. Zhou, G. Hao, P. Thomas, et al., Angew. Chem. Int. Ed. 51 (2012) 10118-10122. DOI:10.1002/anie.201203031 |
| [79] |
X. Chen, H. Zhu, X. Huang, et al., Nanoscale 9 (2017) 2219-2231. DOI:10.1039/C6NR07656D |
| [80] |
C.L. Liu, H.T. Wu, Y.H. Hsiao, et al., Angew. Chem. Int. Ed. 50 (2011) 7056-7060. DOI:10.1002/anie.v50.31 |
| [81] |
Y. Liu, G.F. Tian, X.W. He, et al., J. Mater. Chem. B 4 (2016) 1276-1283. DOI:10.1039/C5TB02322J |
| [82] |
S.K. Sun, L.X. Dong, Y. Cao, et al., Anal. Chem. 85 (2013) 8436-8441. DOI:10.1021/ac401879y |
| [83] |
G. Liang, D. Ye, X. Zhang, et al., J. Mater. Chem. B 1 (2013) 3545-3552. DOI:10.1039/c3tb20440e |
| [84] |
C. Wang, Y. Yao, Q. Song, J. Mater. Chem. C 3 (2015) 5910-5917. DOI:10.1039/C5TC00290G |
| [85] |
E.S. Shibu, S. Sugino, K. Ono, et al., Angew. Chem. Int. Ed. 52 (2013) 10559-10563. DOI:10.1002/anie.201304264 |
| [86] |
W. Hou, F. Xia, G. Alfranca, et al., Biomaterials 120 (2017) 103-114. DOI:10.1016/j.biomaterials.2016.12.027 |
| [87] |
D.H. Hu, Z.H. Sheng, P.F. Zhang, et al., Nanoscale 5 (2013) 1624-1628. DOI:10.1039/c2nr33543c |
| [88] |
C. Xu, Y. Wang, C. Zhang, et al., Nanoscale 9 (2017) 4620-4628. DOI:10.1039/C7NR01064H |
| [89] |
M. Hembury, C. Chiappini, S. Bertazzo, et al., Proc. Natl. Acad. Sci. U. S. A. 112 (2015) 1959-1964. DOI:10.1073/pnas.1419622112 |
| [90] |
D. Shen, M. Henry, V. Trouillet, et al., APL Mater. 5 (2017) 053404. DOI:10.1063/1.4977203 |
| [91] |
R. Jin, C. Zeng, M. Zhou, et al., Chem. Rev. 116 (2016) 10346-10413. DOI:10.1021/acs.chemrev.5b00703 |
| [92] |
S. Wang, X. Meng, A. Das, et al., Angew. Chem. Int. Ed. 53 (2014) 2376-2380. DOI:10.1002/anie.201307480 |
| [93] |
L. Shang, G.U. Nienhaus, APL Mater. 5 (2017) 053101. DOI:10.1063/1.4974514 |
 2018, Vol. 29
2018, Vol. 29