b Tianjin Key Laboratory of Organic Solar Cells and Photochemical Conversion, School of Chemistry and Chemical Engineering, Tianjin University of Technology, Tianjin 300384, China
Polyoxometalates (POMs), a typical class of metal-oxygen clusters constructed from early transition metal cations and oxygen atoms, represent one of the most varied growing areas in inorganic chemistry, and have attracted much attention as their compositional/structural diversity and promising applications [1-9]. As well known, Keggin-type polyoxoanion [α-PMo12O40]3- was initially described by Berzelius in 1826 [10], and then a series of POMs, such as the Keggin, Anderson, Wells–Dawson, Lindqvisttype anions were designed as synthesized [11-13]. Over the past decades, these classical POMs have been widely utilized as inorganic ligands, nodes and/or guest molecules for building transition-metal (TM)-substituted high-nuclear clusters, 1D, twodimensional (2D) and 3D POM-based extended structural materials [14-20]. They have been widely used in the fields of catalysis, optical, electrochemistry, and energy conversion, etc. In comparison, little attention has been paid on the inorganic crown-type Preyssler-type POMs, although they could be used to encapsulate the alkali metal, trivalent lanthanide, and tetravalent actinide cations [21-27]. They have been considered as a potentially useful material in separation of nuclear wastes [21-25]. Up to date, only two members {P5W30O110} and {S5W30O110} (abbreviated as {S5W30}) were explored in the Preyssler-type POM family. Since the first Preysslerpolyoxoanion {P5W30O110} was reported by Preyssler in 1970 [21], and structurally determined by X-ray diffraction 15 years later [22], the Preyssler-type POMs have become a typical structural type of the POM chemistry. Although the {P5W30O110} anion was discovered 40 years ago, few of functional materials were constructed from the {PO4}-based Preyssler anions as their huge volume [27-35]. In 2012, Zhang et al. firstly introduce the {SO4} tetrahedral group into the {W30} crown, preparing a Preyssler-type polyoxoanion {S5W30} [36], which greatly enrich Preyssler–type POM family. However, the modification and functionalization of {S5W30} with the TM, Ln cations and/or metal-organic fragments has never been explored. Therefore, it is a great challenge to make novel extended structural POMs built from {S5W30} unit.
Based on above considerations, we attempt to introduce {S5W30} unit into the inorganic-organic hybrid materials via using TM cations and metal-organic units to decorate the POM precursors. Here, each Preyssler-type {S5W30} was connectedby four dimeric [Ni2(H2O)4(Htrz)3]2 (trz = 1, 2, 4-triazole) metal-organic units into an 1D chain-like structure, which was further packed to form a 3D supramolecular structure via the H-bonding interactions {H[Ni2(H2O)4(Htrz)3]2-(KS5W30O110)} ·18H2O (1). As we known, the sulfate-centered Preyssleranion {S5W30} was firstly functionalized by the TM-organic units, forming the inorganicorganic hybrid materials. Electrocatalytic study indicates that compound 1 exhibits good electrocatalytic activity toward the reduction of H2O2 and NO2-.
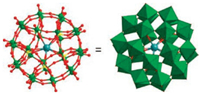
Compound 1 was obtained by reaction of the sulfate-centered Preyssler-type POM{S5W30}, NiCl2·6H2O and triazoleunder the hydrothermal conditions. Single-crystal X-ray diffraction analysis reveals that compound 1 crystallizes in the orthorhombic crystal system, space group Pnnm [37, 38]. It was composed of a Preysslertypeanion {KS5W30O110}9-, two [Ni2(H2O)4(Htrz)3]2 groups and 18 lattice water molecules. The crown-type {KS5W30O110}9- cluster is made up of one {SW6O22} unit and two {S2W12O48} units (Figs. 1 and S1 in Supporting information). The {SW6O22} and {S2W12O48} units both represent the hexavacant units obtained by removing six WO6 octahedra from the saturated Keggin and Wells-Dawsontype polyoxoanions, respectively. As well known, the hexavacant POMs with more coordinated sites have been widely used to assemble TM cations for constructing functionalclusters [39]. In the {S5W30}, the hexavacant {SW6O22} and {S2W12O48} units were fused together through the W-O-W bonds, resulting in the inorganic crown-type polyoxoanion. The cylindrical vacancy of the inorganic crown-type anion {S5W30} captures a K+ ion, resulting in the anion {KS5W30O110}9-.
|
Download:
|
| Fig. 1. (Left) Ball-and-stick and (right) polyhedral representation of Preyssler-type anion {KS5W30O110}9-. Color codes: K azure, W green, S yellow, and O red. | |
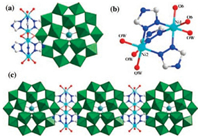
The [Ni2(H2O)4(Htrz)3]2 group consisted of two Ni2+ ions, three Htrz and four coordinated water molecules. In each [Ni2(H2O)4(Htrz)3]2 unit, two nickel centers are fused together by three Htrz ligands forming the dimeric metal-organic unit. As shown in Figs. 2a and b, each Htrz ligand bridges two nickel centers with two adjacent N atoms. The Ni(1) center exhibits a octahedral coordinated environment completed by three nitrogen atoms from three Htrz molecules, two oxygen atoms from two {S5W30} polyoxoanions and one water molecule. The Ni(2) atom was also in a hexa-coordinated environment with three nitrogen atoms from three Htrz molecules, and three water molecules. The Ni–N and Ni– O bond distances are in the ranges of Ni–N = 2.02(5)–2.11(4) Å and 2.02(5)–2.11(3) Å, respectively.
|
Download:
|
| Fig. 2. (a) Polyhedral/ball-and-stick representation of the linking mode between {S5W30} and Ni ions; (b) ball-and-stick representation of [Ni2(H2O)4(Htrz)3]2 in 1; (c) polyhedral/ball-and-stick view of the 1D chain in 1. | |
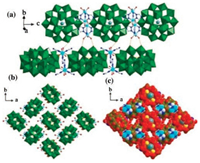
In 1, each anion {S5W30} was connected by four bridging [Ni2(H2O)4(Htrz)3]2 units to construct a 1D straight chain via Ni-O-W bonds (Figs. 2c and S2 in Supporting information), and the Ni-O and W-O bond lengths are 2.11(3) Å and 1.72(3) Å, respectively. By extensive hydrogen bonding interactions between coordinated water molecules of nickel and oxygen atoms on the surface of POMs (Fig. 3a), the 1D chains are further packed together to form a 3D supramolecular structure (Figs. 3b and c). The typical hydrogen bond distances are O17...O27 3.081 Å, O16...O25 3.17 Å, which are responsible for the formation of the final supramolecular network. This 3D framework possess of 1D channel along the caxis with the pore size of 10.6 Å × 5.4 Å.
|
Download:
|
| Fig. 3. (a) 3D supramolecular structure composed of {S5W30}-based 1D chains connected via the H-bonding interactions, (b, c) 3D supramolecular structure of 1. | |
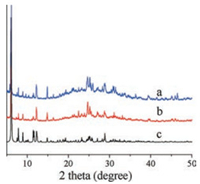
Power X-ray diffraction (PXRD) pattern of compound 1 is showed in Fig. 4. The main peaks of as synthesized sample are consistent with those in the simulated PXRD pattern, indicating the bulk phase purity of the title compound. Further, after immersing the sample in 0.1 mol/L H2SO4 solution for 24 h, there is no obvious change in its PXRD pattern, indicating that the sample is stable in 0.1 mol/L H2SO4 solution. In the IR spectrum (Fig. S4 in Supporting information), the characteristic bands appearing at 1144, 1049, 953, 800, 628, 517, 477 cm-1 can be attributed to the S-O, W=O, W-O-W, and W-O-(S) of {S5W30} anion [36]. The characteristic bands in the range of 1245– 1626 cm-1 are attributed to the typical peaks of the trz ligands. Energy-dispersive X-ray (EDX) analysis shows that the elements W, S, Ni, C, N and K all exist in the {S5W30}-based framework materials (Fig. S3 in Supporting information).
|
Download:
|
| Fig. 4. PXRD patterns of compound 1 (a) after immersing in 0.1 mol/L H2SO4 solution for 24 h, (b) as-synthesized sample, (c) simulated. | |
As well known, POMs are particularly attractive as potential electrocatalysts due to their ability to undergo fast and reversible multi-electron redox process without changing their structures. Compound 1 was obtained by reaction of a huge {S5W30} polyoxoanion with the metal-organic units under hydrothermal condition, which has a low solubility in water and general organic solvents. Therefore, bulk-modified carbon paste electrode (CPE) of compound 1 was prepared and used to investigate its electrochemical and electrocatalytic properties in 0.1 mol/L H2SO4 aqueous solution. In the potential range of -650 mV to 200 mV, there are three reversible redox peaks with the mean peak potentials E1/2 = (Epa+ Epc)/2 = -123, -346 and -505 mV with the scan rate of 100 mV/s, which can be assigned to the redox process of W centersin the {S5W30} anion framework (Fig. 5a). The relationship between the scan rates and the peak currents (Ⅱ) has been researched in the range of 20–400 mV/s. As shown in Fig. 5b, when the scan rates shift from 20 mV/s to 400 mV/s, the peak currents are linearly dependent on the scan rates, indicating that the oxidation-reduction process is a surface controlled process.

|
Download:
|
| Fig. 5. (a) CV of 1-CPE in 0.1 mol/L H2SO4 solution at different scan rates (from inner to outer: 20, 40, 60, 80, 100, 120, 140, 160, 180, 200, 250, 300, 350, 400, 450, and 500 mV/s); (b) Plots of the anodic and cathodic peak Ⅱ–Ⅱ' currents against the scan rates; CVs of 1-CPE in 0.1 mol/L H2SO4 aqueous solution with different concentration of (c) H2O2 and (d) NO2-. Scan rate: 100 mV/s. | |
Hydrogen peroxide and nitrite are very popular in our everyday life, such as they are widely used as the food additive in food factory. The discharge of hydrogen peroxide and nitrite has brought about poisonousness in aquatic biosome. Therefore, removing hydrogen peroxide and nitrite is a significant project and has attracted much attention in the past decades [40]. Herein, electrocatalytic reduction of H2O2 and NaNO2 with 1-CPE was investigated in 0.1 mol/L H2SO4 aqueous solution. Figs. 5c and d show the cyclic voltammetry (CV) curves of 1-CPE accompanied by different concentrations of H2O2 and NaNO2, respectively. With the addition of H2O2 and NaNO2, the reduction peak current drastically increased, while the corresponding oxidation peak currents reduced. These results indicate that compound 1 exhibits electrocatalytic activity for the reduction of H2O2 and NO2-. There are no distinct changes toward the CVs of 1-CPE electrode in the 0.1 mol/L H2SO4 solution after 400 cycles, which reveals the durability of 1-CPE (Fig. S6 in Supporting information).
In conclusion, the sulfate-centered Preyssler-type POM was firstly used to construct extended organic-inorganic hybrid materials. In this structure, the Preyssler-type anion could keep stable under the hydrothermal conditions with a high temperature of 160 ℃, which opens an avenue for constructing the {S5W30}- based extended structural materials. Electrocatalytic study indicates that compound 1 exhibits good electrocatalytic activity toward the reduction of H2O2 and NO2-. In the structure, the Ni(2) center was stabilized by three N atoms from the Htrz ligands in a pyramid geometry and possessed of more exposed active sites occupied by three water molecules, which could be easily replaced by the substrate molecules in the catalytic reaction. Further work is in progress to produce {S5W30}-based 3D open frameworks with Ni (H2O)3 centers.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21671032), Science and Technology Development Project Foundation of Jilin Province (No. 20150520001JH), Science and Technology Research Foundation of the Thirteenth Five Years of Jilin Educational Committee (No. [2015]0056/JJKH20170605KJ).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.12.003.
| [1] |
H.J. Lv, Y.V. Geletii, C.C. Zhao, et al., Chem. Soc. Rev. 41 (2012) 7572-7589. DOI:10.1039/c2cs35292c |
| [2] |
C.H. Zhan, J.M. Cameron, D. Gabb, et al., Nat. Commun. 8 (2017) 14185. DOI:10.1038/ncomms14185 |
| [3] |
S. Yao, H.L. Wu, Z.Q. Lei, J.H. Yan, E.B. Wang, Chin. Chem. Lett. 24 (2013) 283-286. DOI:10.1016/j.cclet.2013.02.008 |
| [4] |
J. Cai, X.Y. Zheng, J. Xie, et al., Inorg. Chem. 56 (2017) 8439-8445. DOI:10.1021/acs.inorgchem.7b01104 |
| [5] |
Y. Zhang, X.B. Han, Z.M. Zhang, Z.J. Liu, E.B. Wang, Chin. Chem. Lett. 24 (2013) 581-584. DOI:10.1016/j.cclet.2013.04.034 |
| [6] |
S.B. Li, L. Zhang, K.P. O'Halloranb, H.Y. Ma, H.J. Pang, Dalton Trans. 44 (2015) 2062-2065. DOI:10.1039/C4DT03625E |
| [7] |
W.J. Luo, J. Hu, H.L. Diao, et al., Angew. Chem. Int. Ed. 56 (2017) 4941-4944. DOI:10.1002/anie.201612232 |
| [8] |
D.Y. Du, J.S. Qin, S.L. Li, Z.M. Su, Y.Q. Lan, Chem. Soc. Rev. 43 (2014) 4615-4632. DOI:10.1039/C3CS60404G |
| [9] |
J. Yu, Y. Cui, C.D. Wu, et al., J. Am. Chem. Soc. 137 (2015) 4026-4029. DOI:10.1021/ja512552g |
| [10] |
J. Berzelius, J. Pogg. Ann. 6 (1826) 369-371. |
| [11] |
J.F. Keggin, Proc. R. Soc. London Ser. A 144 (1934) 75-100. DOI:10.1098/rspa.1934.0035 |
| [12] |
J.S. Anderson, Nature 140 (1937) 850. |
| [13] |
B. Dawson, Acta Cryst. 6 (1953) 113-126. DOI:10.1107/S0365110X53000466 |
| [14] |
Z. Li, X.X. Li, T. Yang, Z.W. Cai, S.T. Zheng, Angew. Chem. Int. Ed. 56 (2017) 2664-2669. DOI:10.1002/anie.201612046 |
| [15] |
J. Dong, J.F. Hu, Y.N. Chi, et al., Angew. Chem. Int. Ed. 56 (2017) 4473-4477. DOI:10.1002/anie.201700159 |
| [16] |
S.T. Zheng, J. Zhang, J.M. Clemente-Juan, D.Q. Yuan, G.Y. Yang, Angew. Chem. Int. Ed. 48 (2009) 7176-7179. DOI:10.1002/anie.v48:39 |
| [17] |
J.C. Liu, J. Yu, Q. Han, et al., Dalton Trans. 45 (2016) 16471-16484. DOI:10.1039/C6DT03148J |
| [18] |
Q.X. Han, B. Qi, W.M. Ren, et al., Nat. Commun. 6 (2015) 10007. DOI:10.1038/ncomms10007 |
| [19] |
Y.W. Liu, S.M. Liu, D.F. He, et al., J. Am. Chem. Soc. 137 (2015) 12697-12703. DOI:10.1021/jacs.5b08273 |
| [20] |
Z.M. Zhang, T. Zhang, C. Wang, et al., J. Am. Chem. Soc. 137 (2015) 3197-3200. DOI:10.1021/jacs.5b00075 |
| [21] |
C. Preyssler, Bull. Soc. Chim. Fr (1970) 30-36. |
| [22] |
M.H. Alizadeh, S.P. Harmalker, Y. Jeannin, J. Martin-Frère, M.T. Pope, J. Am. Chem. Soc. 107 (1985) 2662-2669. DOI:10.1021/ja00295a019 |
| [23] |
K.C. Kim, M.T. Pope, G.J. Gama, M.H. Dickman, J. Am. Chem. Soc. 121 (1999) 11164-11170. DOI:10.1021/ja992105b |
| [24] |
J.A. Fernández, X. López, C. Bo, et al., J. Am. Chem. Soc. 129 (2007) 12244-12253. DOI:10.1021/ja0737321 |
| [25] |
I. Creaser, M.C. Heckel, R.J. Neitz, M.T. Pope, Inorg. Chem. 32 (1993) 1573-1578. DOI:10.1021/ic00061a010 |
| [26] |
C.W. Williams, M.R. Antonio, L. Soderholm, J. Alloys Compd. 303-304 (2000) 509-513. DOI:10.1016/S0925-8388(00)00637-X |
| [27] |
M.H. Dickman, G.J. Gama, K.C. Kim, M.T. Pope, J. Cluster Sci. 7 (1996) 567-583. DOI:10.1007/BF01165802 |
| [28] |
M.R. Antonio, M.H. Chiang, Inorg. Chem. 47 (2008) 8278-8285. DOI:10.1021/ic8008893 |
| [29] |
L. Huang, L. Cheng, S.S. Wang, W.H. Fang, G.Y. Yang, Eur. J. Inorg. Chem. 2013 (2013) 1639-1643. |
| [30] |
X.L. Wang, J. Li, A.X. Tian, et al., Inorg. Chem. Commun. 14 (2011) 103-106. DOI:10.1016/j.inoche.2010.09.042 |
| [31] |
Y. Lu, Y.G. Li, E.B. Wang, X.X. Xu, Y. Ma, Inorg. Chim. Acta 360 (2007) 2063-2070. DOI:10.1016/j.ica.2006.10.045 |
| [32] |
M.X. Liang, C.Z. Ruan, D. Sun, et al., Inorg. Chem. 53 (2014) 897-902. DOI:10.1021/ic4022596 |
| [33] |
Y.Q. Yu, K. Zhao, L.W. Wang, et al., Inorg. Chem. 53 (2014) 11046-11050. DOI:10.1021/ic501567s |
| [34] |
T.P. Hu, Y.Q. Zhao, Z. Jaglicic, et al., Inorg. Chem. 54 (2015) 7415-7423. DOI:10.1021/acs.inorgchem.5b00962 |
| [35] |
A. Hayashi, H. Ota, X. Lopez, et al., Inorg. Chem. 55 (2016) 11583-11592. DOI:10.1021/acs.inorgchem.6b02116 |
| [36] |
Z.M. Zhang, S. Yao, Y.G. Li, et al., Chem. Eur. J. 18 (2012) 9184-9188. DOI:10.1002/chem.v18.30 |
| [37] |
G. M. Sheldrick, SHELXS 97, Program for Crystal Structure Solution, University of Göttingen, Göttingen, Germany, 1997.
|
| [38] |
G. M. Sheldrick, SHELXL 97, Program for Crystal Structure Refinement, University of Göttingen, Göttingen, Germany, 1997.
|
| [39] |
D.D. Zhang, Z.J. Liang, S.Q. Xie, et al., Inorg. Chem. 53 (2014) 9917-9922. DOI:10.1021/ic501575x |
| [40] |
X.L. Wang, R. Zhang, X. Wang, et al., Dalton Trans. 46 (2017) 1965-1974. DOI:10.1039/C6DT04185J |
 2018, Vol. 29
2018, Vol. 29 


