b Department of Chemistry, University of Michigan, Ann Arbor MI 48109, United States;
c Department of Orthopedic, Nanjing Jinling Hospital, Nanjing 210096, China
Magnetite (iron oxide, Fe3O4) particles are commonly employed particles for biomedical applications [1-3]. Interactions of magnetite particles with cells play key roles in executing their biological functions and in determining their toxicity. When particles are executing their biological functions, they encounter complex extracellular biomolecules (e.g., proteins) which adsorb to their surface to form a biomolecule-corona which would affect the particle-cell interactions [4-10]. Endocytosis, the way that particles are transported into cells, is the initial step of particlecell interactions. Autophagy, an essential catabolic process that ultimately results in either cell's survival or death, is a cellular outcome of invaded particles [11-15]. Therefore, information concerns the endocytosis and the autophagic effects of magnetite particles is crucial for understanding the particle-cell interactions.
As early as 1984, flow cytometric light scatter analysis has been used for evaluating the efficacy of immunogold particles on cell surface labeling [16]. More than two decades later, Yuko Ibuki's group clarified the flow cytometric light scatter analysis for evaluating the cellular uptake potential of nanoparticles [17]. A few years later, R.M. Zucker's group confirmed the effective of the above method for detecting the cellular uptake of silver, and TiO2 nanoparticles, and they also used light scattering based microscopy for observing the internalized particles [18, 19]. Light scattering based analyses for evaluating and observing the cellular uptake of particles is simple yet effective, however, reports concerned the combination of their application was rarely.
In this work, we investigated the effects of bovine serum proteins on the endocytosis of magnetite spherical particles (MSPs). Autophagic effects of MSPs in breast cancer cells were studied. Light scattering based flow cytometry and microscopy were used for evaluating the uptake potential of MSPs by cells and the cellular autophagosome accumulation
Magnetite spherical particles (MSPs) were synthesized by solvent-thermal reaction with trisodium citrate (Na3Cit) as an electrostatic stabilizer according to a previous report [20]. Magnetite particles were spherical and approximately 190 nm in diameter (Fig. S1 in Supporting information). Average hydrodynamic diameter of MSPs was 207.0 ± 3.4 nm (Fig. S2 in Supporting information). Average hydrodynamic diameter of MSPs was 351.2 ± 34.3 nm in serum-free medium (SF) or Dulbecco's high glucose modified Eagle's medium and 361.8 ± 17.2 nm in serumcontaining medium (cDMEM) or Dulbecco's high glucose modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum medium (Fig. S3 in Supporting information). ζ-Potential of MSPs was -14.9 ± 0.3 mV in SF and -10.8 ± 1.1 mV in cDMEM. Data are presented as mean ± standard deviation of three measurements. Average hydrodynamic diameter of MSPs increased in SF compared with that in ultrapure water. We consider this effect was a result of MSPs aggregating in cell culture medium as it was reported that the citrate ligands, which bonded to the surfaces of MSPs when Na3Cit was used as an electrostatic stabilizer, would desorb from the particle surface and preferentially complexed by chemical species present in the culture medium [21]. Hydrodynamic diameter of MSPs in cDMEM was about 10 nm larger than that in SF, and ζ-potential of MSPs in cDMEM also increased to a value closer to electric neutrality. We consider these effects were due to adsorption of proteins to MSPs' surfaces in the presence of serum proteins, and these happened to iron oxide [22], gold [23], silica [4] and polystyrene [7] nanoparticles as well.
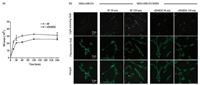
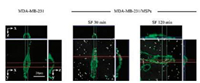
In order to investigate the effects of serum proteins on the cellular uptake of MSPs, MDA-MB-231 cells were continuously incubated with MSPs in SF or cDMEM at 37 ℃. Cells were harvested for flow cytometry analysis, average side-scattered light intensity (SS mean) of flow cytometry increased rapidly at first and then slowly, ultimately reached to a constant (Fig. 1a). However, compared to "SF" group, SS mean in "cDMEM" group increased more slowly and reached to a lower value (Fig. 1a). Results indicated that the cellular uptake of MSPs was increased with the exposing time and the efficiency of MSPs was decreased in the presence of serum proteins. This is in good agreement with previous reports that show decreased cellular uptake of polymer [6], gold [24], and silica particles [4], to various extents, due to the presence of serum proteins. Cells incubated with MSPs were further incubated with cholesterol-PEG-FITC, and their scattering light (white spot) and fluorescence (green) observed by confocal laser scanning microscope (CLSM) was combined for visually confirming the cellular uptake of MSPs. MSPs possess the property of scattering visible light, thus they can be distinguished from cells by CLSM with specific beam-splitter. As shown in Fig. 1b, several white spots were found in light scattering field of MDA-MB-231 cells incubated with MSPs in SF or cDMEM for 30 min, and it increased when cells were further incubated with MSPs for 120 min. Compared to "SF", cells incubated with MSPs in cDMEM possess less white spots (Fig. 1b), indicating the cellular uptake amounts of MSPs were decreased in the presence of serum proteins. The results were consistent with that of flow cytometric analysis. Light scattering based analyses were proved to be effective and their experimental procedures were much simpler than conventional methods. Cellular uptake procedures of MSPs were investigated by three dimensional images as shown in Fig. 2, there were clusters of MSPs adsorbed to the membrane of MDAMB-231 cells incubated with MSPs in SF for 30 min. When cells were further incubated with MSPs for 120 min, there were several MSPs located at the cytoplasm (Fig. 2), indicating the endocytosis of MSPs had at least two steps: particles adsorbed to cell membrane and particles were engulfed by cells.
|
Download:
|
| Fig. 1. Effects of serum proteins on the uptake of MSPs by MDA-MB-231 cells. (a) Average SS mean based flow cytometric analysis. Error bars are standard deviations of three independent experiments. (b) Visually confirming the uptake of MSPs by confocal laser scanning microscopy. Cells were incubated with MSPs in SF or cDMEM for different time. Scattering lights of MSPs are shown as white spots and cell membranes are green. | |
|
Download:
|
| Fig. 2. Visually confirming the procedure of cellular uptake of MSPs by 3D images. MDA-MB-231 cells were incubated with MSPs in SF for 30 min or 120 min. MSPs are shown as white spots and cell membranes are green. | |
In order to investigate the effects of serum proteins on the cellular adsorption of MSPs, cells were cultured with MSPs in SF or cDMEM at 4 ℃. Cells were harvested for flow cytometry analysis, side-scattered light of flow cytometry decreased significantly when MDA-MB-231 cells were cultured with MSPs at 4 ℃ (Fig. 3a) compared with that at 37 ℃, indicating the uptake of MSPs by MDA-MB-231 cells was energy dependent. The results were in agreement with previous reports that endocytosis of iron oxide nanoparticles was energy dependent and their internalization by cells was prevented at 4 ℃ [25, 26]. SS mean of "cDMEM" group reduced by 94.8% compared to that in "SF" (Fig. 3a), indicating serum proteins greatly affected the cellular adsorption of MSPs. Scattering light (white spot) and fluorescence (green) observed by CLSM were combined for visually observing the cellular adsorption of MSPs. Much more white spots were in MDA-MB-321 cells in "SF" group compared to that in "cDMEM" (Fig. 3b). The results were in agreement with that of flow cytometric analysis. We consider the initial decreasing of cellular adsorption of MSPs contributed to the reduced uptake efficiency.

|
Download:
|
| Fig. 3. Effects of serum proteins on the cellular adsorption of MSPs. (a) Average SS mean based flow cytometric analysis. Error bars are standard deviations of three independent experiments. (b) Visually confirming the adsorption of MSPs by confocal laser scanning microscopy. MDA-MB-231 cells were cultured with MSPs in SF or cDMEM at 4 ℃ for 1 h. MSPs are shown as white spots and cell membranes are green. | |
Endocytic pathways of particles have been extensively studied by using pharmacological inhibitors [27-29]. In the present work, chlorpromazine (CPZ), genistein, methyl-β-cyclodextrin (MβCD) and 5-(N, N-dimethyl) amiloride hydrochloride (DMA) were used for identifying the possible internalization pathway of MSPs by MDA-MB-231 cells. Inhibitors exhibited weaker effect on the cellular uptake of MSPs in cDMEM compared to that in SF (Fig. 4). Cellular uptake of MSPs in "SF" group was significantly inhibited (by more than half) by CPZ, which was an clathrin-mediated endocytosis inhibitor, and slightly inhibited by genistein and MβCD, which were caveolin-mediated endocytosis inhibitors. Differently, DMA, which was macropinocytosis inhibitor, somewhat facilitate the cellular uptake of MSPs in "cDMEM" group (Fig. 4). Clearly, in addition to reducing the cellular adsorption of MSPs, serum proteins also had influences on the endocytic mechanisms of MSPs. It was likely that MSPs were transported into cells by clathrin-mediated endocytosis in the absence of serum proteins, however, this should be further studied using siRNA depletion or knock-out of clathrin or adaptor protein (AP-2), and a quantitative analysis co-localization of particles with markers of clathrin-coated structures, which is beyond the scope of this research.

|
Download:
|
| Fig. 4. Effects of serum proteins on the endocytic pathway of MSPs. MDA-MB-231 cells were pre-treated with inhibitors: CPZ, genistein, MβCD or DMA, and then cells were incubated with MSPs in SF or cDMEM. "Control" was MDA-MB-231 cells incubated with MSPs while haven’t been pre-treated with inhibitors. | |
In order to study the cellular autophagosome accumulation induced by MSPs, cells incubated with MSPs were further incubated with CYTO-ID® autophagy detection dye for 20 min before CLSM observation. There were few autophagosomes in MDA-MB-231 cells of regular incubation (Fig. 5). Autophagosomes accumulated in MDA-MB-231 cells of starved culturing (Fig. 5), which has been known was a result of autophagy activation [14]. As shown in Fig. 5, a few autophagosomes were found in MDA-MB- 231 cells incubated with MSPs for 2 h, and it increased dramatically when cells were further incubated with MSPs for 24 h, their amount was even more than that in cells of starved culturing, indicating MSPs had remarkably effects on cell autophagy. Interestingly, when cells were incubated with MSPs for 2 h, we found MSPs adsorbed to cell membrane and there was no autophagosome in cell as shown in the rectangle region further magnified at the bottom (Fig. 5). When cells were incubated with MSPs for 24 h, the internalized MSPs were almost located at or nearby the autophagosomes as shown in the rectangle region further magnified at the bottom (Fig. 5). Results show us the accumulation of autophagosomes was a cellular response to the internalized particles rather than the membrane bound ones. A recent report supported nanoparticles undergoing ubiquitination similar to pathogens [30], and autophagy activation was regarded as an attempt of cells to increase their total degradative capacity in order to eliminate the invaded NPs [31, 32]. Refer to above previous reports, we consider the autophagosome accumulation in MDAMB-231 cells may be an attempt of cells to sequester and eliminate the internalized MSPs, which were perceived by cells as foreign or aberrantobjects, similar to bacteria and other pathogens. After incubated with MSPs for 24 h, several cells exhibited accumulation of autophagosomes while nucleus vanished as yellow arrows pointed, implying that these cells were suffering autophagic death.

|
Download:
|
| Fig. 5. Effects of MSPs on the autophagosome accumulating in MDA-MB-231 cells. MDA-MB-231 cells were regular cultured ("Regular") or cultured under serum-free medium ("Starved"). MDA-MB-231 cells were cultured with MSPs ("MDA-MB-231/MSPs") for 2 h ("2 h") and 24 h ("24 h"), respectively. The rectangle region is further magnified at the bottom. The yellow arrow points the cells with accumulation of autophagosomes while with nucleus vanished. MSPs are shown as white dots, autophagosomes are green and nucleus are blue. | |
In conclusion, light scattering based analyses, included sidescattered light of flow cytometry and light scattering based microscopy, were used for evaluating the effects of serum proteins on cellular uptake of MSPs and the cellular autophagosome accumulation induced by MSPs. Light scattering based microscopy was combined with fluorescence of cell organelles (membrane, nucleus or autophagosome), for localizing the MSPs in cells. Sidescattered light of flow cytometric analysis was applicable to visually detect the endocytosis of various metal-based nanoparticles with diameter larger than 20 nm. These general methods were proved to be simple yet effective, and the present work may promote their application in studies upon endocytosis of metallic particles in the future. Fundamental findings in the present work, which concerned the endocytosis and autophagic effects of MSPs, promoted our understanding upon the interactions of MSPs with cells, and will contribute to better applications of magnetite based particlesin biology and medicine.
AcknowledgmentsWe acknowledge Prof. Martin Philbert in University of Michigan, School of Public Health, helping us with the cell experiments, Dr. Xianbo Mou in Ningbo University, offering the magnetite spherical particles; Z. Chen acknowledges the support from the University of Michigan. The National Natural Science Foundation of China (Nos. 61527806), National Key Research and Development Program of China (No. 2017YFA0205301), Natural Science Foundation of Jiangsu Province (No. BK20141397), National Key Program for Developing Basic Research (No. 2014CB744501), Research Fund for the Doctoral Program of Higher Education of China (No. 20120092120042), CMA-L'Oreal China Skin Grant 2015 (No. S2015121421) are also acknowledged for the financial support.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.11.017.
| [1] |
T. Schlorf, M. Meincke, E. Kossel, et al., Int. J. Mol. Sci. 12 (2011) 12-23. |
| [2] |
A. Akbarzadeh, M. Samiei, S. Davaran, Nanoscale Res. Lett. 7 (2012) 144. DOI:10.1186/1556-276X-7-144 |
| [3] |
N. Raschzok, C.M. Langer, C. Schmidt, et al., Cell Transplant 22 (2013) 1959-1970. DOI:10.3727/096368912X661382 |
| [4] |
A. Lesniak, F. Fenaroli, M.P. Monopoli, et al., ACS Nano 6 (2012) 5845-5857. DOI:10.1021/nn300223w |
| [5] |
F. Wang, L. Yu, M.P. Monopoli, et al., Nanomedicine 9 (2013) 1159-1168. DOI:10.1016/j.nano.2013.04.010 |
| [6] |
Y. Yan, K.T. Gause, M.M.J. Kamphuis, et al., ACS Nano 7 (2013) 10960-10970. DOI:10.1021/nn404481f |
| [7] |
D. Walczyk, F.B. Bombelli, M.P. Monopoli, I. Lynch, K.A. Dawson, J. Am. Chem. Soc. 132 (2010) 5761-5768. DOI:10.1021/ja910675v |
| [8] |
L. Wang, J. Li, J. Pan, et al., J. Am. Chem. Soc. 135 (2013) 17359-17368. DOI:10.1021/ja406924v |
| [9] |
A. Musyanovych, V. Fetz, S. Tenzer, et al., Nat. Nanotechnol. 8 (2013) 772-781. DOI:10.1038/nnano.2013.181 |
| [10] |
C.C. Fleischer, C.K. Payne, Acc. Chem. Res. 47 (2014) 2651-2659. DOI:10.1021/ar500190q |
| [11] |
Y. Yu, J. Duan, Y. Yu, et al., Toxicol. Res. 5 (2016) 871-882. DOI:10.1039/C5TX00465A |
| [12] |
V.R. Lopes, V. Loitto, J.N. Audinot, et al., J. Nanobiotechnol. 14 (2016) 22. DOI:10.1186/s12951-016-0174-0 |
| [13] |
K.N. Yu, T.J. Yoon, A. Minai-Tehrani, et al., Toxicol. In Vitro 27 (2013) 1187-1195. DOI:10.1016/j.tiv.2013.02.010 |
| [14] |
X. Ma, Y. Wu, S. Jin, et al., ACS Nano 5 (2011) 8629-8639. DOI:10.1021/nn202155y |
| [15] |
A.R. Mishra, J. Zheng, X. Tang, P.L. Goering, Toxicol. Sci. 150 (2016) 473-487. DOI:10.1093/toxsci/kfw011 |
| [16] |
R.M. Bohmer, N.J. King, Cytometry 5 (1984) 543-546. DOI:10.1002/(ISSN)1097-0320 |
| [17] |
H. Suzuki, T. Toyooka, Y. Ibuki, Environ. Sci. Technol. 41 (2007) 3018-3024. DOI:10.1021/es0625632 |
| [18] |
R.M. Zucker, K.M. Daniel, E.J. Massaro, et al., Cytometry Part A 83 (2013) 962-972. |
| [19] |
R.M. Zucker, E.J. Massaro, K.M. Sanders, L.L. Degn, W.K. Boyes, Cytometry Part A 77A (2010) 677-685. DOI:10.1002/cyto.a.v77a:7 |
| [20] |
J. Liu, Z. Sun, Y. Deng, et al., Angew. Chem. Int. Ed. 48 (2009) 5875-5879. DOI:10.1002/anie.v48:32 |
| [21] |
M. Sa, J. Courtois, M. Seigneuret, H. Conjeaud, J. Berret, Biomaterials 32 (2011) 9353-9363. DOI:10.1016/j.biomaterials.2011.08.048 |
| [22] |
E.M. Luther, C. Petters, F. Bulcke, et al., Acta Biomater. 9 (2013) 8454-8465. DOI:10.1016/j.actbio.2013.05.022 |
| [23] |
M.A. Dobrovolskaia, A.K. Patri, J. Zheng, et al., Nanomedicine 5 (2009) 106-117. DOI:10.1016/j.nano.2008.08.001 |
| [24] |
X. Cheng, X. Tian, A. Wu, et al., ACS Appl. Mater. Interfaces 7 (2015) 20568-20575. DOI:10.1021/acsami.5b04290 |
| [25] |
J.S. Kim, T.J. Yoon, K.N. Yu, et al., J. Vet. Sci. 7 (2006) 321-326. DOI:10.4142/jvs.2006.7.4.321 |
| [26] |
M. Geppert, M.C. Hohnholt, K. Thiel, et al., Nanotechnology 22 (2011) 145101. DOI:10.1088/0957-4484/22/14/145101 |
| [27] |
L. Wang, Y. Liu, W. Li, et al., Nano Lett. 11 (2011) 772-780. DOI:10.1021/nl103992v |
| [28] |
H. Yang, S.Y. Fung, M. Liu, Angew. Chem. Int. Ed. 50 (2011) 9643-9646. DOI:10.1002/anie.201102911 |
| [29] |
C. Petters, R. Dringen, Neurochem. Int. 81 (2015) 1-9. DOI:10.1016/j.neuint.2014.12.005 |
| [30] |
S.T. Stern, P.P. Adiseshaiah, R.M. Crist, Part. Fibre Toxicol. 9 (2012) 20. DOI:10.1186/1743-8977-9-20 |
| [31] |
S.J. Soenen, J. Demeester, S.C. De Smedt, K. Braeckmans, Nano Today 8 (2013) 121-125. DOI:10.1016/j.nantod.2012.12.001 |
| [32] |
K. Peynshaert, B.B. Manshian, F. Joris, et al., Chem. Rev. 114 (2014) 7581-7609. DOI:10.1021/cr400372p |
 2018, Vol. 29
2018, Vol. 29 


