b Beijing Jialin Pharmaceutical Co., Ltd., Beijing 100121, China;
c Center for Imaging and Systems Biology, Minzu University of China, Beijing 100081, China
Chronic obstructive pulmonary (COPD) as a progressive lung disease has received widespread attention for its high rates of mortality and morbidity [1, 2]. Bronchodilators mainly divided into antimuscarinic agents, β2 adrenergic agonist and methylxanthines, play an important role in the management of COPD [3-5]. Arformoterol (ARF), N-{2-hydroxy-5-[(1R)-1-hydroxy-2-{[(2R)-1-(4-methoxyphenyl)propan-2-yl]amino}ethyl] phenyl}formamide was the first long-acting β2 adrenergic agonist approved by the U.S. Food and Drug Administration (FDA) for nebulized delivery, thus offering a new option for patients who are unable to use drypowder inhalation [6-9]. It presented consistent improvements in lung function for patients with COPD.
A comprehensive profiling of pharmacokinetic parameters and the features of tissue distribution are essential for uncovering the in vivo behaviors and functional mechanism of drugs [10, 11]. Inhalation therapy is promising for respiratory disease, due to its rapid and non-invasive characteristic [12]. It is capable of delivering drugs directly to the site of action with immediate pharmacological effects and minimum side effects [13, 14]. And thus it is necessary to evaluate ARF pharmacokinetic and tissue distribution in respiratory system. However, to our knowledge, there is only one report on the pharmacokinetic study of ARF in human [9], which focused on comparing pharmacodynamic and pharmacokinetic effects between ARF tartrate inhalation solution and formoterol dry powder inhaler in COPD patients with no chromatography, mass spectrometry and validation details presented. And there is neither reported tissue distribution study nor LC–MS/MS methods that allow for a precise quantitative analysis of ARF in respiratory system (mainly including lung and trachea).
To address this, a rapid and sensitive UPLC–MS/MS method was developed and validated for the determination of ARF in rat plasma and respiratory organs (lung and trachea), respectively. The lower limit of quantification (LLOQ) was found to be 1.83 pg/mL for plasma and 3.90 pg/mL for lung and trachea homogenates. Pharmacokinetic profiles of ARF after inhalation administration of arformoterol tartrate inhalation solution (50 μg/kg) were assessed by the developed UPLC–MS/MS method. Furthermore, the lung and trachea tissue distribution of ARF were also demonstrated in this research. The in vivo behavior information obtained from this study will facilitate future exploration on ARF and even other inhalation agents.
For picogram per milliliter-level ARF in plasma, high sensitivity and specificity LC-ESI-MRM is essential for the detectable and reliable quantitative results. ARF demonstrated a great sensitivity in positive ion mode by directly infused into ion source via a syringe pump. Key parameters like declusting potential (DP), collision energy (CE) and gas parameters were optimized to achieve a better sensitivity. Typical MS/MS mass spectra and the fragmentation pathway of ARF and internal standard (IS, tulobuterol) were illustrated in Fig. 1. The ion pair of m/z 345.2 ([M+H]+ → m/z 149.1 with CE (25 V)) was chosen as MRM transition for ARF. Tulobuterol was selected as IS for its similar chromatographic behavior with ARF, but since the relative strong precursor ion m/z 154.0 presented a poor signal-to-noise ratio, MRM transition of 228.1 ([M+H]+ → m/z 172.1 with CE (20 V)) was finally selected for IS. The specific parameters for each analyte are displayed in Table S1 (Supporting information).

|
Download:
|
| Fig. 1. MS/MS mass spectra and the fragmentation pathway of ARF (A) and IS (B) in positive ion mode. | |
Acetonitrile produced higher MS response and lower background noise than methanol and thus was chosen as the organic phase. The sensitivity and reproducibility of ARF increased substantially when 0.1% formic acid was added in aqueous phase. The separation was firstly performed under isocratic conditions. However, although no matrix effect of ARF was observed in plasma sample, the matrix effect was significant in lung and trachea tissue homogenates, especially for continuous assay, which may be caused by abundant and strong retention nonpolar phospholipids metabolites in respiratory organs [15-18]. Thus, gradient elution start with 75% proportion of aqueous phase was set to elute polar molecules, and then 100% organic phase was introduced to elute nonpolar endogenous molecules. The optimized gradient elution achieve a great peak shape and sensitivity with negligible matrix effect, and ensure more than 500 measurements of bio-samples successfully performed using one analytical column.
The effect of different extraction methods including liquidliquid extraction (LLE) and protein precipitation (PPT) were investigated. LLE was adopted in this study for its sensitivity and better peak shape. After comparing different extraction solvents (methyl tert-butyl ether, diethyl ether-cyclohexane (4/1, v/v) and ethyl acetate), ethyl acetate was chosen owing to the relative high extraction recovery. The specificity of this method was confirmed and representative MRM chromatograms of blank plasma, blank lung and trachea homogenate samples, blank biosamples spiked with ARF and IS were showed in Fig. 2. The retention time of ARF and IS were both at 2.0 min with no obvious interferences of endogenous entities.

|
Download:
|
| Fig. 2. Representative MRM chromatograms of ARF (Ⅰ) and IS (Ⅱ): (A) blank rat plasma sample, (B) blank rat plasma sample spiked with ARF and IS, (C) blank lung and trachea homogenate, (D) blank lung and trachea homogenate spiked with ARF and IS. | |
Over the concentration range of 1.83–458 pg/mL for ARF in plasma, 3.90–1560 pg/mL in lung and trachea homogenate samples, the calibration curves presented a great linearity, with a correlation coefficient (R2) > 0.9980 and 0.9988, respectively. The typical regression equation curves were: y = 0.054x + 0.00403 for plasma samples, y = 0.0139x + 0.00254 for tissue homogenate samples. The RSD values on the slope were with ±15%. LLOQ were 1.83 pg/mL and 3.90 pg/mL for plasma and tissue homogenates, respectively. In the beginning, serious carry-over effects were observed after analysis of upper limit of quantification samples. Since, acetonitrile-1% aqueous formic acid (8/2, v/v) was set as strong wash solvents and isopropanol-1% aqueous formic acid (2/8, v/v) was introduced as weak wash solvents, carry-over effects were eliminated.
The intra- and inter-day precision and accuracy were established from validation runs performed at LLOQ and three quality control (QC) concentrations (Table S2 in Supporting information). The intra- and inter-day precision (RSD) of ARF were within the range of 5.2%–13.2% for plasma, and 4.6%–8.1% for tissue homogenates. As for the intra- and inter-day accuracy, relative error (RE) values were no more than 11.0% in plasma and 8.1% or less in tissue homogenates, which all were within the acceptable range. The mean extraction recoveries of ARF at three QC levels were 90.5%, 91.9% and 103.9% for rat plasma samples, while 79.5%, 79.3% and 76.9% for rat lung and trachea tissue homogenates (Table S2). Mean recovery for IS (1.0 ng/mL in plasma and 4.0 ng/mL in tissue homogenates) were 90.0% and 77.3%, respectively. Matrix effects values varied from 96.7% to 111.9% with a maximum RSD = 4.4% for plasma and from 93.2% to 96.4% with RSD < 2.9%. The matrix effect of IS (1.0 ng/mL in plasma and 4.0 ng/mL in tissue homogenates) were 92.3% and 100.6%, respectively.
Stability assessment of ARF in plasma and tissue were designed to cover anticipated storage conditions for preprocessing of the bio-samples. Details were presented in Table S3 (Supporting information). The results suggest that no significant variation of ARF in bio-samples after 18 h at room temperature, 5 h in autosampler, dried residue in room temperature for 20 h, three freeze-thaw cycles and stored at -20 ℃ for 2 weeks. Besides, stock solutions of ARF and IS were remain stable after stored at 4 ℃ for 21 days and kept in room temperature for 6 h.
For pharmacokinetic study, 6 rats (3 males and 3 females) inhalation administration of arformoterol tartrate inhalation solution (ATIS) at dose of 50 μg/kg (Detail information was illustrated in Supporting information). About 200 μL blood samples were collected before administration, 0.17, 0.33, 0.5, 0.75, 1, 2, 4, 8, 12, 16 and 24 h after dosing. All blood samples were centrifuged immediately at 3000 rpm for 5 min to obtain plasma. As for lung and trachea distribution study, 18 rats were divided into 3 groups (3 males and 3 females each group). After inhalation administration of ATIS (50 μg/kg), the lung tissue and the trachea tissue were removed immediately at 0, 0.5 and 1 h, thoroughly rinsed and homogenized in 0.9% saline solution.
The concentration-time curve of ARF in rat plasma after inhalation administration of ATIS (50 μg/kg) was illustrated in Fig. 3A. Pharmacokinetic parameters were calculated with DAS 3.2.7 software (Drug and Statistics, Shanghai, China) and demonstrated in Table S4 (Supporting information). Cmax of ARF was 101.0 ± 82.0 pg/mL with the elimination half-life (t1/2) of 2.3 ± 0.6 h. For AUC(0-t) and AUC(0-∞) values were 99.0 ± 63.0 pg h/mL and 107.0 ± 61.0 pg h/mL, respectively. CLz/F was 617.2 ± 348.0 L h-1 kg-1. For MRT(0-t) and MRT(0-∞) values were 1.9 ± 0.4 h and 2.7 ± 0.5 h. The concentration of ARF in lung and trachea tissue homogenates immediately (0 h), 0.5 h and 1 h after inhalation administration of ATIS (50 μg/kg) were showed in Fig. 3B. The data suggest that 3.48 ± 3.63 ng/g, 1.55 ± 0.40 ng/g and 1.09 ± 0.37 ng/g ARF in lung tissue at 0 h, 0.5 h and 1 h were detected, while the content of ARF in trachea tissue were 2.59 ± 1.85 ng/g, 1.94 ± 0.93 ng/g, and 1.24 ± 0.89 ng/g at corresponding time. Furthermore, 1 h after administration, lung and trachea tissue still demonstrated a certain amount of ARF, which be consistent with the relative stable content of ARF in plasma at 0.33 h to 1.0 h.
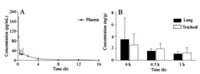
|
Download:
|
| Fig. 3. (A) The concentration-time curve of ARF in rat plasma. (B) The content of ARF in lung and trachea tissue after inhalation administration of ATIS (50 μg/kg). | |
In summary, a rapid and sensitive UPLC–MS/MS method was developed and fully validated for analysis of ARF in rat plasma, rat lung and trachea tissue homogenates. To the best of our knowledge, this is the first validated LC–MS/MS method for the quantification of ARF in biological samples. The method offers extremely sensitivity (LLOQ of 1.83 pg/mL for plasma and 3.90 pg/mL for lung and trachea tissue homogenates) and was proved to be precise, reproducible and robust for the analysis of ARF. Moreover, the sensitive method was successfully applied to the pharmacokinetic, lung and trachea tissue distribution study after inhalation administration of ATIS to rats. The data suggest that ARF can be rapidly absorbed into blood through respiratory systems, lung and trachea tissue maintain a certain amount of ARF in 1 h after administration. The information obtained from this work will benefit the further studies on ARF.
AcknowledgmentsWe would like to thank Beijing Jialin Pharmaceutical Co., Ltd. for offering Arformoterol tartrate and Arformoterol tartrate inhalation solution. This research was supported by CAMS Innovation Fund for Medical Sciences (No. 2016-I2M-1-009) and National Scientific and Technological Major Project for New Drugs (No. 2017ZX09101003-002-004).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.01.015.
| [1] |
J.L. Lopez-Campos, W. Tan, J.B. Soriano, Respirology 21 (2016) 14-23. |
| [2] |
M. Miravitlles, C. Vogelmeier, N. Roche, et al., Respir. J. 47 (2016) 625-637. DOI:10.1183/13993003.01170-2015 |
| [3] |
M.G. Matera, J. Ora, M. Cazzola, Ther. Clin. Risk Manag. 11 (2015) 1805-1811. |
| [4] |
Y.S. Punekar, S.H. Landis, K. Wurst, H. Le, Respir. Res. 16 (2015) 141. DOI:10.1186/s12931-015-0295-2 |
| [5] |
D. Singh, Br. J. Clin. Pharmacol. 79 (2015) 695-708. DOI:10.1111/bcp.12545 |
| [6] |
M.C. Miles, J.F. Donohue, J.A. Ohar, Ther. Adv. Respir. Dis. 7 (2013) 81-86. DOI:10.1177/1753465812465784 |
| [7] |
C.H. Loh, J.F. Donohue, J.A. Ohar, Expert Opin. Drug Saf. 14 (2015) 463-472. DOI:10.1517/14740338.2015.998196 |
| [8] |
V. Ganapathy, M.D. Stensland, Int. J. Chronic Obstruct. Pulmon. Dis. 12 (2017) 1793-1801. DOI:10.2147/COPD |
| [9] |
J. Kharidia, C.M. Fogarty, C.F. Laforce, et al., Pulm. Pharmacol. Ther. 21 (2008) 657-662. DOI:10.1016/j.pupt.2008.03.003 |
| [10] |
P. Zhao, G. Gao, L. Zhang, et al., J. Pharm. Biomed. Anal. 141 (2017) 262-269. DOI:10.1016/j.jpba.2017.03.036 |
| [11] |
Y. Liu, X. Xun, J. Yi, Y. Xiang, J. Hua, Chin. Chem. Lett. 28 (2017) 1093-1098. DOI:10.1016/j.cclet.2016.11.026 |
| [12] |
P. Agent, H. Parrott, Breathe (Sheff) 11 (2015) 110-118. DOI:10.1183/20734735.021014 |
| [13] |
K. Togami, Y. Kanehira, H. Tada, Biopharm. Drug Dispos. 36 (2015) 205-215. DOI:10.1002/bdd.v36.4 |
| [14] |
N. Barapatre, P. Symvoulidis, W. Moller, et al., J. Pharm. Biomed. Anal. 102 (2015) 129-136. DOI:10.1016/j.jpba.2014.09.001 |
| [15] |
O. Gonzalez, M.E. Blanco, G. Iriarte, et al., J. Chromatogr. A 1353 (2014) 10-27. DOI:10.1016/j.chroma.2014.03.077 |
| [16] |
N. Kadian, K.S. Raju, M. Rashid, et al., J. Pharm. Biomed. Anal. 126 (2016) 83-97. DOI:10.1016/j.jpba.2016.03.052 |
| [17] |
M. Griese, H.G. Kirmeier, G. Liebisch, et al., PLoS One 10 (2015) e0117985. DOI:10.1371/journal.pone.0117985 |
| [18] |
K.A. Zemski Berry, R.C. Murphy, B. Kosmider, R.J. Mason, J. Lipid Res. 58 (2017) 926-933. DOI:10.1194/jlr.M074955 |
 2018, Vol. 29
2018, Vol. 29 


