b State Key Laboratory of Environmental Chemistry and Ecotoxicology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China
Bisphenol S (BPS), a major alternative to bisphenol A (BPA), has estrogenic, androgenic, antiestrogenic and antiandrogenic activity similar to BPA [1]. BPS also displayed DNA damage and cellular dysfunction from an in vitro study [1]. However, the molecular mechanisms of BPS-induced hepatic toxicity in vivo are still unclear. Abnormal metabolism plays a very important role in toxicity study of environmental pollutants. Metabolomics is a useful tool for the identification of potential markers both in vitro and in vivo [2-5]. Lipids that are a branch of metabolites play regulator and transmitter in cell proliferation and apoptosis [2-5].
Mass spectrometry (MS) based lipid analysis in situ provides very useful information to elucidate the variation of tissue microenvironment upon the pollutant exposure et al. [7] found hepatitis B virus could cause lipid alteration in composition and metabolism following the acute infection and regeneration of mouse liver. They identified phosphatidylcholines (PCs) composition displaying remarkable vary by using matrixassisted laser desorption/ionization (MALDI)-mass spectrometry imaging (MSI) in mouse liver. Wang et al. [8] developed a workflow of N-(1-naphthyl)-ethylenediamine dihydrochloride (NEDC)-assisted laser desorption/ionization time-of-flight mass spectrometry (LDI-TOF) MSI to realize in situ label-free analysis of multiple GPs in the mouse model of colorectal cancer liver metastasis. Arts et al. [9] explored the dynamic molecular changes of amino acid by measuring the dilution and conversion of stable isotopes. MSI method showed the dynamics of L-phenylalanine metabolism in mouse model. In our study, we used an integrated liquid chromatography-mass spectrometry (LC-MS)/MS and MALDIMSI to evaluate hepatic toxicity of BPS in mouse model, which may provide a new insight to elucidate potential bisphenolsinduced metabolic diseases.
The animal experiment procedures have been described in our previous report, in which kidney toxicity was investigated [10]. Briefly, female BALB/c nude mice aged 4-weeks-old were randomly divided into two groups (n = 6 for each) including control group (olive oil) and BPS-treated group (100μg BPS/kg body weight/day). BPS dosage was adjusted for body weight daily. The 100μg BPS/kg body weight/day dose was selected because it appeared to induce some adverse effects in our previous studies (such as inflammation, renal toxicity and tumor proliferation; data not shown). In addition, the dose of BPS is far below the toxicological lowestobserved-effect-level (LOEL) of 20 mg/kg [1]. All mice were treated by gastric infusion method with a dosage of BPS in olive oil once daily. After 56 days consecutive administration of olive or BPS, the mice were sacrificed and the liver samples were collected for omics analysis by using LC-MS/MS and MALDI-MSI. Livers were harvested within 20 min from nude mice and gallbladders were removed. Livers were washed by PBS buffer and frozen in liquid nitrogen, and then were stored at -80 ℃ until time of detection.
The details of experiment for lipidomics research were described in previous work by Zhao et al. [11]. The MALDI-MSI analytical procedures were same as those reported by Wang et al. [8] and Liu et al. [12]. Briefly, fresh liver tissue was frozen at -20 ℃, and then sectioned and thaw-mounted onto indium tin oxide (ITO) coated glass slides and dried. Solutions of NEDC as matrix and mixture of standards were prepared as described by Wang et al. [8]. The experiments were performed on a rapifleXTM MALDI TissuetyperTM (Bruker Daltonics) equipped with a smartbeamTM 3D laser in the single focus mode [10]. The mass spectra data were acquired at a mass range of m/z 200-1100 in the negative reflector ion mode by averaging signal from 1000 shots at 3.0 × 2810 volts detector gain and 30% laser power. MALDI-MSI raw data and ion images were first opened in flexImaging 5.0 software (Bruker Daltonics), and then analyzed, calibrated and imported into SCiLS Lab 2016a software. The standard processing pipeline was used for statistical analysis of MSI data with probabilistic latent semantic analysis (pLSA).
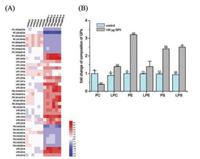
Based on hormonal or physiological effect studies of BPS in vivo, we selected the BPS exposure level of 100μg BPS/kg body weight/day in this study [1]. To evaluate whether BPS exposure has an effect on liver lipidome, we developed a scheme to investiagte accurate and exaustive lipid profiles of liver tissue by combining LC-Orbitrap Fusion Tribird and LipidSearch software for automated identification of lipids. The identified differential lipids were detected according to partial least squares discriminant analysis (PLS-DA) by using LipidSearch, SIEVE and SIMCA-P software (Table S1 in Supporting information and Fig. 1A). As shown in Fig. 1A, glycerophospholipid (GP) metabolites, including phosphatidylethanolamines (PE), lysophosphatidylcholines (LPC), lysophosphatidylethanolamines (LPE) and lysophosphatidylserine (LPS) up-regulated significantly, as well as phosphatidylcholine (PC) and phosphatidylserine (PS) down-regulated remarkably in liver tissues from the BPS-treated mice (P < 0.05). Our results demonstrated that BPS exposure perturbed lipidome of liver significantly in the mouse model.
|
Download:
|
| Fig. 1. BPS exposure altered GP composition in mouse liver. The composition of major GP including PC, LPC, PE, LPE, PS and LPS were analyzed by LC-MS/MS. (A) Heat map analysis of identified lipids after BPS exposure. The individual samples were represented in the vertical axis, and the identified lipids were represented in the horizontal axis. Up- and down-regulated lipids were represented in red and blue in BPS-treated group vs. control group. (B) Relative composition of subclass lipids (lipid compositional reprogramming)% = amount of subclass/entire lipidome × 100% (n = 6). BPS-treated/control, fold change of BPS-treated and control group (the symbol represents statistical significance between treated group and control group: * P < 0.05, ** P < 0.01). | |
To study whether BPS exposure has an effect on lipid composition we analyzed the relative proportion of each GP subclasses in liver tissues of both control and BPS-treated mice by using LC-MS/MS. As shown in Fig. 1B, liver GP composition was significantly different between the control and BPS-treated groups. The composition of PC (with 0.4-fold decrease) were lower and PE (with 3.2-fold increase) were higher in 100μg BPS-treated group compared to control group (P < 0.05). It has been reported that the PC/PE ratio plays critical role in the cellular membrane function associated with cell proliferation and disease generation [13, 14]. Lipid distribution in cellular membrane have asymmetrically characteristics across the bilayer within the choline-containing PC in the outer leaflet and the amine-containing PE localized in the inner leaflet [15]. Upon the micro-environmental stimuli, insufficient PC or decrease PC/PE ratio could cause variation of lipid distribution and could trigger various toxic effects, such as cell apoptosis and proliferation [16, 17]. We speculated that a decrease PC/PE ratio could induce the perturbation of membrane homeostasis, which might change lipid affinity as substrates of enzymes and cause hepatic injury [18].
To investigate whether BPS exposure could change GP distribution in liver tissues, we used NEDC as matrix to realize GP imaging by combining MALDI-MSI analysis with SCiLS Lab software. Based on the MSI data, a spatial segmentation of the entire set of liver sections was performed in control and the BPStreated group (Fig. 2). The spatial segmentation analysis showed that different regions could share the common molecular features. As shown in Fig. 2A, control (blue) & BPS-treated group had significant differences in lipid spatial distribution (P < 0.05). From the above spatial segmentation analysis, four molecularly different clusters were identified among the liver tissues including the blue region in control group, the red, orange and yellow regions in the BPS-treated group. In order to screen the potential markers related to BPS treatment, pLSA was performed from the two groups including control and BPS groups by using SCiLS Lab software. As shown in Fig. 2B, pLSA displayed a visual distinction according to detected ions of two groups. Six differential lipids were screened in liver tissues. Ion intensities of GPs including PE (20:1/20:4), LPC (20:4), LPS (33:4) and LPE (20:4) increased gradually in the order of control and 100 μg BPS groups. However, GPs including PC (20:4/ 22:6) and PS (18:0/22:6) showed lower ion intensities in the BPStreated group compared to control group (P < 0.05). Above all, MSI analysis was employed to confirm not only alteration of lipid ion intensities but also specificity of lipid spatial distribution in liver tissues of the BPS-treated mice.

|
Download:
|
| Fig. 2. MALDI-MSI analysis of BPS-induced GP fatty acid remodeling in mouse liver, including (A) imaging of GP classes, spatial segmentation and co-localization of MALDI-MSI data; (B) pLSA score plots for MALDI-MSI profiles obtained from the liver sections (n = 6); Control and 100mg BPS treatment were represented in green and red, respectively. | |
GP plays very important role as structural components of cellular membrane and as precursors of lipid mediators [19]. In the biosynthesis of GP pathway, diversity of acyl groups of GP include the esterification of saturated and monounsaturated fatty acid at sn-1 position, as well as esterification of polyunsaturated acyl groups at sn-2 position [20, 21]. GPs are modified through the turnover of sn-2 acyl moiety of GPs in fatty acid remodeling pathway. These remodeling processes are attributed to the coordinated actions of lysophospholipid acyltransferases and phospholipase A2 s [20, 21]. In our study, BPS exposure significantly induced GP fatty acid remodeling from global metabolic processes. The proposed GP remodeling network in liver tissues of the BPStreated mice is shown in Fig. 3.

|
Download:
|
| Fig. 3. BPS-induced GP fatty acid remodeling in mouse liver. Up- and downregulated lipids and genes (italics) were represented in red and green, respectively. Representative images of BPS-treated liver section in GP metabolism pathway were displayed in negative reflector ion mode. | |
To explore the factor of altered GP, the expression kinetics of enzymes that regulate GP fatty acid remodeling was analyzed. Mouse mRNA of LPC acyltransferase 3 (LPCAT3), LPCAT4, LPE acyltransferase 1 (LPEAT1), LPEAT2, PLA2G2A and PLA2G12A were highly expressed in both BPS-treated groups. The mRNA expression of LPCAT1, LPCAT2 and LPS acyltransferase (LPSAT) downregulated in both BPS-treated groups compared to the control group (Table S2 in Supporting information). LPCAT1 exhibits LPG acetyltransferase (LPGAT) activity by using 18:2- or 18:3-acyl-CoA, and catalyzes dipalmitoyl-PC synthesis. In addition, LPCAT2 possesses not only LPCAT activity using 20:4-CoA as donor in membrane homeostasis but also lyso platelet-activating factor (PAF) acetyltransferase activity in the biosynthesis of PAF. LPCAT3 has activities of LPCAT, LPEAT and LPSAT. It displayed higher activities with 20:4 and 18:2-CoA than those with saturated fatty acyl-CoA. LPCAT4 possesses LPCAT and LPEAT activities using with 18:1-CoA as donors [22, 23]. Moreover, LPEAT1 exhibits the LPEAT, LPCAT, LPSAT and LPGAT activities with 20:4- and 18:1-CoA [24]. Thus, we speculated that BPS exposure could cause the variation of enzyme activity in liver tissues, which might result in the GP fatty acid remodeling and cause instability of cellular membrane and generation of hepatic injury.
Our results demonstrated that the liver lipidome of BPS-treated mouse with an abundance variation of lipids included the upregulated PE, LPC, LPE, LPS, and down-regulated PC and PS linked with fatty acid remodeling, leading to an abnormal constitutive proportion of PC, PE and PS, and an aberrantly expression of enzymes related to GP metabolism. Further molecular mechanism is needed to explore the direct role of fatty acid remodeling on lipid metabolism in liver tissues upon the BPS exposure.
AcknowledgmentsThe work was supported by the grants from the National Natural Science Foundation of China (Nos. 21507106, 91543202), Hong Kong Research Grants Council-General Research Fund (No. 1230195) and Hong Kong Baptist University Strategic Development Fund (No. 15-1012-P04).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.01.034.
| [1] |
J.R. Rochester, A.L. Bolden, Environ. Health Perspect. 123 (2015) 643-650. |
| [2] |
N. McCarthy, Nat. Rev. Cancer 12 (2012), doi: http://dx.doi.org/10.1038/nrc3248.
|
| [3] |
Z.H. Zhang, N.D. Vaziri, F. Wei, et al., Sci. Rep. 6 (2016) 22151. DOI:10.1038/srep22151 |
| [4] |
C. Stegemann, R. Pechlaner, P. Willeit, et al., Circulation 129 (2014) 1821-1831. DOI:10.1161/CIRCULATIONAHA.113.002500 |
| [5] |
J.C. Han, J. Yu, Y.J. Gao, Pharm. Biol. 52 (2014) 1624-1628. DOI:10.3109/13880209.2014.900810 |
| [6] |
J.L. Norris, R.M. Caprioli, Chem. Rev. 113 (2013) 2309-2342. DOI:10.1021/cr3004295 |
| [7] |
E.S. Par, J.H. Lee, J.H. Hong, et al., Plos One 9 (2014) e103955. DOI:10.1371/journal.pone.0103955 |
| [8] |
J.N. Wang, S.L. Qiu, C.Q. Xiong, et al., Anal. Chem 87 (2015) 422-430. DOI:10.1021/ac504294s |
| [9] |
M. Arts, Z. Soons, S.R. Ellis, et al., Angew. Chem. 129 (2017) 1-6. DOI:10.1002/ange.v129.1 |
| [10] |
C. Zhao, P. S. Xie, Y. Y. Song, et al., 15th International Conference on Environmental Science and Technology, Rhodes, Greece, 31 August to 2 September, 2017.
|
| [11] |
C. Zhao, Z. Tang, J.C. Yan, et al., Sci. Total Environ. 592 (2017) 357-365. DOI:10.1016/j.scitotenv.2017.03.035 |
| [12] |
H.H. Liu, W. Li, Q. He, et al., Sci. Rep. 7 (2017) 41954. DOI:10.1038/srep41954 |
| [13] |
P. Fagone, S. Jackowski, Biochim. Biophys. Acta 1831 (2013) 523-532. DOI:10.1016/j.bbalip.2012.09.009 |
| [14] |
Z. Li, L.B. Agellon, T.M. Allen, et al., Cell Metab. 3 (2006) 321-331. DOI:10.1016/j.cmet.2006.03.007 |
| [15] |
G. van Meer, D.R. Voelker, G.W. Feigenson, Nat. Rev. Mol. Cell Biol. 9 (2008) 112-124. DOI:10.1038/nrm2330 |
| [16] |
C.L. Yen, M.H. Mar, S.H. Zeisel, FASEB J. 13 (1999) 135-142. DOI:10.1096/fasebj.13.1.135 |
| [17] |
A. Lykidis, S. Jackowski, Prog. Nucleic Acid Res. Mol. Biol. 65 (2001) 361-393. |
| [18] |
H. Shindou, D. Hishikawa, T. Harayama, K. Yuki, T. Shimizu, J. Lipid Res. (2009) S46-S51. |
| [19] |
S. Ishii, T. Shimizu, Prog. Lipid Res. 39 (2000) 41-82. DOI:10.1016/S0163-7827(99)00016-8 |
| [20] |
A. Yamashita, T. Sugiura, K. Waku, J. Biochem. 122 (1997) 1-16. DOI:10.1093/oxfordjournals.jbchem.a021715 |
| [21] |
W.E. Lands, Biochim. Biophys. Acta 1483 (2000) 1-14. DOI:10.1016/S1388-1981(99)00177-8 |
| [22] |
H. Shindou, T. Shimizu, J. Biol. Chem. 284 (2009) 1-5. DOI:10.1074/jbc.R800046200 |
| [23] |
P.P. Robichaud, K. Boulay, J.É. Munganyiki, et al., J. Lipid Res. 54 (2013) 2665-2677. DOI:10.1194/jlr.M037044 |
| [24] |
A. Yamashita, Y. Hayashi, N. Matsumoto, et al., Biology (Basel) 3 (2014) 801-830. |
 2018, Vol. 29
2018, Vol. 29 


