b Faculty of Geosciences and Environmental Engineering, Southwest Jiaotong University, Chengdu 610031, China
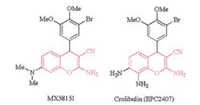
2-Amino-4H-chromene is a class of privileged structural motif frequently appearing in natural products and pharmaceutically active compounds [1]. In particular, 2-amino-4H-chromene possessing a cyano group at the 3-position attracted considerable attention due to its powerful antitumor activity [1a, 2]. Among all these compounds, 2-amino-3-cyano-4H-chromenes containing an aryl group at the 4-position were identified as potent apoptosis inducers [3]. These compounds were found to be potent inhibitors of tubulin polymerization as well and to effectively inhibit the binding of colchicine to tubulin [3]. MX-58151 (Fig. 1) retained activity in tumor cells resistant towards current antimitotic agents, taxanes (including Taxol and Taxotere) and Vinca alkaloids [2a, 3-4]. Therefore, it represented a new class of potential therapeutic agent for the treatment of drug-resistant cancer. In addition, several 4-aryl-4H-chromenes were also determined to be effective vascular disrupting agents (VDA) [2b, 5]. One of the lead VDA candidates, Crolibulin (Fig. 1), also known as EPC2407, is currently in phase Ⅰ/Ⅱ clinical trials [2b]. Notably, the antitumor activity of Crolibulin is closely correlated with its absolute configuration. It is revealed that the R-isomer is approximately 50-100 times more active than its S-enantiomer [6].
|
Download:
|
| Fig. 1. Structures of the pharmaceutically active moleculars. | |
Considering these appealing pharmaceutical activities, elegant approaches have been proposed to prepared these enantioenriched 2-amino-3-cyano-4H-chromene [7]. However, to the best of our knowledge, current protocol mainly focuses on the synthesis of 4-alkyl-substituted ones [7a-g]. The catalytic enantioselective synthesis of 4-aryl chromenes was relatively less explored [7h-n]. Meanwhile, the reported strategies for the synthesis of 4-aryl chromenes suffered from several drawbacks such as unsatisfactory enantiopurity [7i, k] and specialized substrate scope [7h, 7j-m]. Therefore, developing a highly enantioselective and general strategy for the construction of chiral 2-amino-3-cyano-4-aryl-4H-chromene is still highly desirable.
para-Quinone methides (p-QMs) have been increasingly utilized as a class of prevailing Michael acceptor for 1, 6-conjugate additions [8]. As a result, a range of diarylmethine compounds were obtained with high degrees of optical purities [9]. In contrast, the functionalized p-QMs have been less explored and the related domino reaction has not been well investigated, despite the great potential in the construction of complex diarylmethine-containing natural product and functional molecules [10]. In this context, Enders group presented a subtle domino oxa-Michael/1, 6-conjugated addition process in a highly stereoselective manner, employed the functionalized ortho-hydroxyl-substituted p-QMs [11]. More recently, Jiang and his co-operator utilized this versatile precursor to construct densely functionalized spiro[chromane-2, 1'-isochromene] scaffold in an achiral fashion [12]. Regretfully, the tedious prior preparation procedure of this functionalized starting material might limit their wide application. Based on our continuous effort in the asymmetric organocatalytic cascade reactions [7g], herein we would like to present a highly efficient tandem 1, 6-conjugate addition/annulation/tautomerization of functionalized p-QMs, during the preparation of this paper, a similar strategy was applied by Li et al., [13]. A variety of 2-amino-3-cyano-4-aryl-4H-chromenes were afforded with high optical purities. Notably, the functionalized ortho-hydroxyl-substituted p-QMs could be feasibly accessed via one-step transformation (see Supporting information), using the simple commercially available precursor.
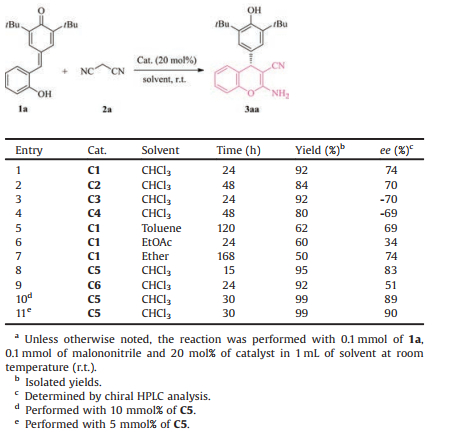
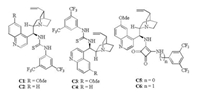
We commenced our investigation with utilizing ortho-hydroxyl p-QM 1a and malononitrile 2a as the model substrates. Initially, the catalytic effect of various bifunctional thiourea based on cinchona alkaloids [14] was evaluated in chloroform. Gratifyingly, catalysts C1-C4 (Fig. 2) properly promoted the designed 1, 6-conjugated addition/annulation/tautomerization domino process, and the desired 4-aryl-substituted chromene 3aa was generated in acceptable yield and promising optical purity (Table 1, entries 1-4). Further solvent screening revealed that chloroform was the favorable one in terms of reactivity and enantioselectivity (Table 1, entries 5-7 vs. entry 1). In order to further improve the enantioselectivity, the quinine-based squaramides C5 and C6 were then investigated. Notably, an obvious enhancement of enantiomeric purity was observed in the presence the squaramide C5 [15] in comparison with the corresponding thiourea C1 (Table 1, entry 8 vs. entry 1). The quinine-based squaramide C5 proved to be the preferred catalyst in view of reactivity and enantioselectivity (Table 1, entry 8 vs. entry 9). The catalyst loading exerted an impact on this cascade process to a certain extent. The model reaction proceeded equally efficiently in the presence of diminished amount of squaramide C5, albeit slightly prolonged time was required (Table 1, entries 10 and 11). In contrast, superior enantiomeric excess (90% ee) was achieved in the presence of 5 mol% of C5 (Table 1, entry 11 vs. entries 8 and 10).
|
Download:
|
| Fig. 2. The structures of bifunctional organocatalysts. | |
|
|
Table 1 Optimization of reaction conditions.a |
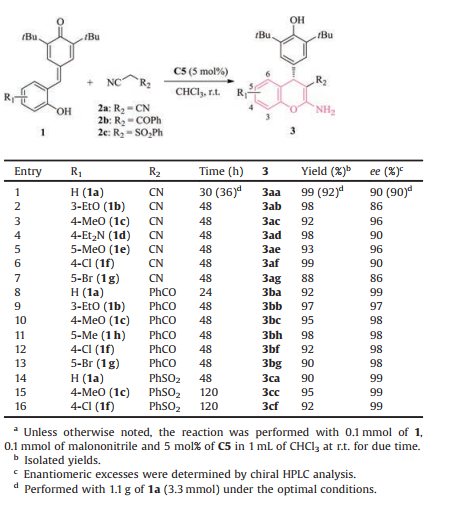
Having established the optimal reaction conditions, we successively surveyed the generality and scope of this tandem 1, 6-conjugate addition/annulation/tautomerization process of various p-OMs with a range of cyano-containing compounds (Table 2). For electron-rich p-OMs 1b-e, the titled cascade process occurred smoothly and afforded the desired chromenes 3ab-ae in excellent yields and satisfactory enantiopurities (Table 2, entries 2-5). The sterical hindrance of substituent on the aromatic ring had a slight influence on the enantioselectivity of this asymmetric process. The sterically more bulky 3-ethoxyl-substituted 1b offered 3ab with relatively poorer enantioselectivity (86% ee) (Table 2, enty 2). This domino reaction was independent of the electronic property of the substituent on the benzene ring. In accordance with the electron-neutral and electron-rich p-OMs 1a-e, the electron-deficient p-OMs 1f and 1 g were all well tolerated, giving rise to the expected chromenes 3af and 3ag with 90% and 86% ee, respectively (Table 2, entries 6 and 7). Expect malononitrile, benzoylacetonitrile 2b was also well compatible with this catalytic system. Notably, the replacement of cyano group with benzoyl group led to especially high optical purities in the case of p-OMs with various electronic nature (97%-99% ee) (Table 2, entries 8-13). Moreover, (phenylsulfonyl)acetonitrile 2c readily underwent the tandem reaction, producing the expected 4-aryl-chromenes 3ca-cf with almost perfect enantiocontrol (99% ee), albeit prolonged reaction time (120 h) was required in contrast with malononitrile 2a and benzoylacetonitrile 2b (Table 2, entries 14-16). To test the synthetic utility of this established protocol, the model reaction was performed on a gram-scale. To our delight, the desired product 3aa was smoothly obtained in 92% yield and maintaining enantiomeric purity, albeit slightly prolonged reaction time (36 h) was required (Table 2, entry 1). In addition to ortho-hydroxyl p-QM 1a, we have made attempt to prepare ortho-aminosubstituted analogues. However, the compound of this type could not be obtained via our protocol at present.
|
|
Table 2 Substrate scopes.a |
The absolute configuration of product 3 was unambiguously determined to be S via single crystal X-ray analysis of compound 3af, and the crystallographic data has been deposited in the Cambridge Crystallographic Data Centre (CCDC 1572731). Other products were assigned by analogy (Scheme 1). According to the above-mentioned results, a plausible reaction pathway was proposed to account for the product formation (Scheme 1). In the initial step, the 1, 6-Michal addition between p-OMs and malononitrile gave rise to the adduct A in the presence of bifunctional squaramide C5. The subsequent intramolecular oxanucleophilic addition to the cyano group constructed the cyclic chromane motif. Finally, intermediate B underwent tautomerization to generate the desired 4-aryl chromene 3af. As described in Scheme 1, squaramide C5 associated with p-OM and malononitrile to form ternary complex through hydrogen-bonding. The hydrogen bonds orientated the reactants in the proper position, resulting in preferential attack of malononitrile toward the Si-face of p-OM. This proposed transition state was in good agreement with the absolute configuration of product.
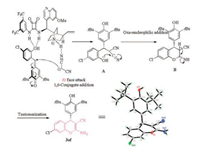
|
Download:
|
| Scheme 1. A plausible reaction pathway. | |
In summary, a novel cascade process initiated by 1, 6-conjugated addition has been successfully developed to prepare pharmaceutically active 2-amino-4-aryl-4H-chromenes. The cascade reaction proceeded smoothly under mild conditions and furnished the desired products in high yields (88%–99%) and excellent enantiopurities (86%–99% ee). A broad combination of p-OMs with various cyano-containing compounds was well tolerated by this protocol. The starting material, functionalized ortho-hydroxyl-substituted p-OMs, could be more facilely obtained in comparison with early reported procedure [11]. The expansion of these functionalized p-OMs to other domino reactions is well underway in our lab.
AcknowledgmentsThis work is financially supported by the National Natural Science Foundation of China (No. 21402163) and the Fundamental Research Funds for the Central Universities of Southwest Minzu University (No. 2016NGJPY02). Cong Duan gratefully acknowledges the Graduate Innovation Project of Southwest Minzu University (No. CX2017SZ016). Wenqin Xu acknowledges the Undergraduate Innovation Project of Southwest Minzu University (No. S201610656092). Professor Fengrong Dai of Fujian Institute of Research on the Structure of Matter is greatly acknowledged for Xray crystal structure determination.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.11.044.
| [1] |
(a) S. A. Patil, R. Patil, L. M. Pfeffer, et al., Future Med. Chem. 5 (2013) 1647-1660; (b) J. L. Wang, D. Liu, Z. J. Zhang, et al., Proc. Natl. Acad. Sci. U. S. A. 97 (2000) 7124-7129. |
| [2] |
(a) S. Kasibhatla, H. Gourdeau, K. Meerovitch, et al., Mol. Cancer Ther. 3 (2004) 1365-1374; (b) S. X. Cai, J. Drewe, W. Kemnitzer, Anticancer Agents Med. Chem. 9 (2009) 437-456. |
| [3] |
W. Kemnitzer, J. Drewe, S. Jiang, et al., J. Med. Chem. 47 (2004) 6299-6310. DOI:10.1021/jm049640t |
| [4] |
H. Gourdeau, L. Leblond, B. Hamelin, et al., Mol. Cancer Ther. 3 (2004) 1375-1384. |
| [5] | |
| [6] |
S.X. Cai, A. J., Drewe, S. Kasibhatla, et al., Cai S.X., J.A., Drewe, Kasibhatla S., et al 7 (2011) 968, 595 B2. |
| [7] |
(a) J. W. Xie, X. Huang, L. P. Fan, et al., Adv. Synth. Catal. 351 (2009) 3077-3082; (b) Y. Gao, W. Yang, D. M. Du, Tetrahedron: Asymmetry 23 (2012) 339-344; (c) G. Yang, C. Luo, X. Mu, et al., Chem. Commun. 48 (2012) 5880-5882; (d) W. Li, H. Liu, X. Jiang, et al., ACS Catal. 2 (2012) 1535-1538; (e) K. Hu, A. Lu, Y. Wang, et al., Tetrahedron: Asymmetry 24 (2013) 953-957; (f) W. Li, J. Huang, J. Wang, Org. Biomol. Chem. 11 (2013) 400-406; (g) Y. Q. Zheng, C. F. Luan, Z. J. Wang, et al., Chin. Chem. Lett. 27 (2016) 25-30; (h) A. Adili, Z. L. Tao, D. F. Chen, et al., Org. Biomol. Chem. 13 (2015) 2247-2250; (i) X. S. Wang, G. S. Yang, G. Zhao, Tetrahedron: Asymmetry 19 (2008) 709-714; (j) W. Chen, Y. Cai, X. Fu, et al., Org. Lett. 13 (2011) 4910-4913; (k) Y. Gao, D. M. Du, Tetrahedron: Asymmetry 24 (2013) 1312-1317; (l) B. Wu, X. Gao, Z. Yan, et al., Tetrahedron Lett. 56 (2015) 4334-4338; (m) B. Wu, X. Gao, Z. Yan, et al., Org. Lett. 17 (2015) 6134-6137; (n) L. Caruana, M. Mondatori, V. Corti, et al., Chem. -Eur. J. 21 (2015) 6037-6041; (o) Q. Ren, W. Y. Siau, Z. Du, et al., Chem. -Eur. J. 17 (2011) 7781-7785. |
| [8] |
(a) A. Parra, M. Tortosa, ChemCatChem 7 (2015) 1524-1526; (b) L. Caruana, M. Fochi, L. Bernardi, Molecules 20 (2015) 11733; (c) P. Chauhan, U. Kaya, D. Enders, Adv. Synth. Catal. 359 (2017) 888-912. |
| [9] |
(a) W. D. Chu, L. F. Zhang, X. Bao, et al., Angew. Chem. Int. Ed. 52 (2013) 9229-9233; (b) L. Caruana, F. Kniep, T. K. Johansen, et al., J. Am Chem. Soc. 136 (2014) 15929-15932; (c) Z. Wang, Y. F. Wong, J. Sun, Angew. Chem. Int. Ed. 54 (2015) 13711-13714; (d) Y. Lou, P. Cao, T. Jia, et al., Angew. Chem. Int. Ed. 54 (2015) 12134-12138; (e) F. S. He, J. H. Jin, Z. T. Yang, et al., ACS Catal. 6 (2016) 652-656; (f) K. Zhao, Y. Zhi, A. Wang, et al., ACS Catal. 6 (2016) 657-660; (g) X. Z. Zhang, Y. H. Deng, X. Yan, et al., J. Org. Chem. 81 (2016) 5655-5662; (i) X. Li, X. Xu, W. Wei, et al., Org. Lett. 18 (2016) 428-431; (j) Y. F. Wong, Z. Wang, J. Sun, Org. Biomol. Chem. 14 (2016) 5751-5754; (k) C. Ma, Y. Huang, Y. Zhao, ACS Catal. 6 (2016) 6408-6412; (l) L. Ge, X. Lu, C. Cheng, et al., J. Org Chem. 81 (2016) 9315-9325; (m) C. Jarava-Barrera, A. Parra, A. López, et al., ACS Catal. 6 (2016) 442-446; (n) N. Dong, Z. P. Zhang, X. S. Xue, et al., Angew. Chem. Int. Ed. 55 (2016) 1460-1464; (o) T. C. Kang, L. P. Wu, Q. W. Yu, et al., Chem. -Eur. J. 23 (2017) 6509-6513; (p) L. Roiser, M. Waser, Org. Lett. 19 (2017) 2338-2341; (q) S. Li, Y. Liu, B. Huang, et al., ACS Catal. 7 (2017) 2805-2809. |
| [10] |
(a) X. Z. Zhang, Y. H. Deng, K. J. Gan, et al., Org. Lett. 19 (2017) 1752-1755; (b) X. Z. Zhang, K. J. Gan, X. X. Liu, et al., Org. Lett. 19 (2017) 3207-3210. |
| [11] |
K. Zhao, Y. Zhi, T. Shu, et al., Angew. Chem. Int. Ed. 55 (2016) 12104-12108. DOI:10.1002/anie.201606947 |
| [12] |
S. Liu, X.C. Lan, K. Chen, et al., Org. Lett. 19 (2017) 3831-3834. DOI:10.1021/acs.orglett.7b01705 |
| [13] |
L. Zhang, X. Zhou, P. Li, et al., RSC Adv. 7 (2017) 39216-39220. DOI:10.1039/C7RA08157J |
| [14] |
B.J. Li, L. Jiang, M. Liu, et al., Synlett (2005) 603-606. |
| [15] |
J.P. Malerich, K. Hagihara, V.H. Rawal, J. Am. Chem. Soc. 130 (2008) 14416-14417. DOI:10.1021/ja805693p |
 2018, Vol. 29
2018, Vol. 29