With the reactive C=N bond, imines are widely used in various organic transformations such as reduction, addition, cyclization and aziridination reactions [1]. They are also versatile intermediates for the synthesis of pharmaceuticals, particularly for the preparation of anti-inflammatory agents, non-natural amino acids and anticancer agents [2]. Therefore, synthesis and application of imines is essentially ever-appealing research field in chemistry.
The typical process for the preparation of the imines involves the condensation of primary amines with carbonyl compounds, especially odorous and unstable aldehydes, whereas this protocol requires acid-promotes in many situations [3]. During the past decade, new methodologies for the synthesis of imines have been developed, such as the oxidative coupling of amines with the greener and more available alcohols, the self-coupling of primary amines and the oxidative dehydrogenation of secondary amines. A wide variety of catalysts including metal catalysts [4], metal-free catalysts [5], photo-catalysts [6] and bioinspired catalysts [7] have been extensively explored and bring significant developments to these new methodologies. In these reported methods, the direct synthesis of imines by oxidative coupling of alcohols and amines is very attractive because diverse symmetric and asymmetric imines can be easily synthesized by choosing different starting substrates [8]. However, some drawbacks still remained to be solved satisfactorily: (1) Stoichiometric oxidants, which will produce large amounts of undesired waste, were needed in the early methods; (2) Not easily accessed catalysts, noble metal complexes, or sensitive and expensive ligands were essential in some cases; (3) Harsh reaction conditions could not be avoided sometimes; (4) Dangerous pure oxygen was used in some aerobic oxidation reactions. These drawbacks restricted the practical applications of imines formation from alcohols and amines.
Recent years, nitroxyl radicals have been applied to synthesis of imines. Several catalyst systems such as CuI/Bipy/TEMPO [9], Cu(OAc)2/DMAP/TEMPO [10], Pd(OAc)2/Et3N/TEMPO/t-BuOK [11] and Fe(NO3)3/TEMPO/KOH [12] were successfully developed for the oxidative coupling of alcohols with amines. Nevertheless, these catalytic systems still require transition metal catalysts, which might thereby increase the risk for traces of metals in the products, and limit their applications in the field of pharmaceutical chemistry. Hence, the development of a transition-metal-free and practical catalytic system for the oxidative coupling of alcohols with amines is still highly desirable.
Inspired by hereinbefore work, as a part of our research program aimed at transition-metal-free nitroxyl-catalyzed oxidation reactions [13], we reported an efficient methodology for synthesis of imines from alcohols and amines with air as the oxidant and the synthetically accessible 9-azabicyclo[3.3.1]nonan-N-oxyl (ABNO) as the catalyst.
ABNO, keto-ABNO and ABNOH were prepared in our laboratory according to the literatures [14, 15]. Other chemicals were purchased from supplier and used without any further treatment. 1H NMR spectra were recorded on 500 MHz; 13C NMR spectra were recorded on 125 MHz. CDCl3 was used as the solvent with tetramethylsilane (TMS) as the internal standard. GC analyses were conducted with a flame ionization detector (FID) and a DB-5 or FFAP capillary column. GC–MS (EI, 70 eV) was performed with DB-5 MS capillary column. Melting points were measured using melting point instrument and are uncorrected. General procedure for the synthesis of imines: To a sealed tube equipped with a magnetic stir, was added aniline (1.0 mmol), alcohol (1.2 mmol, 1.2 equiv.), ABNO (4.2 mg, 0.03 mmol, 3 mol%), KOH (16.8 mg, 0.3 mmol, 30 mol%) and toluene (0.5 mL). Then air was introduced via a balloon. The mixture was stirred at 80 ℃ until the reaction was complete (determined by GC or TLC). The mixture was directly purified through column chromatography using petroleum and Et3N (100:1) as eluent to afford the pure product.
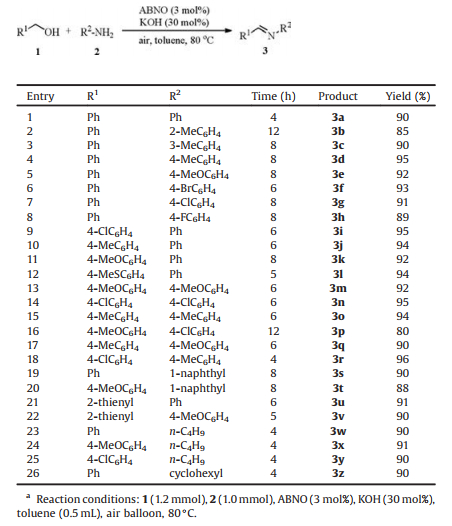
We started to optimize the reaction conditions for the oxidative coupling of alcohols and amines to form the corresponding imines using benzyl alcohol (1a) and aniline (2a) as the model substrates (Table 1). Initially, the reaction was carried out in toluene with 3 mol% of ABNO under atmosphere pressure of air in balloon at 80 ℃, and about 1.3% yield of N-benzylideneaniline (3a) could be observed (entry 1). According to Zhao et al. [16], stoichiometric KOH could promote imines formation from alcohols and amines. To our delight, when 30 mol% of KOH was added, the yield of 3a was increased to 92% (entry 2). Other nitroxyl radicals such as TEMPO, 4-OH-TEMPO, 4-AcNH-TEMPO and keto-ABNO were also investigated, and the results displayed that ABNO/KOH was the most efficient catalyst combination (entries 2–6). Then different ABNO/base combinations were tested. The reactions in the presence of NaOH and t-BuONa provided moderate yields (entries 7 and 8), while very low yield was obtained in the presence of K2CO3 (entry 10). Though good result could be achieved with t-BuOK (entry 9), it was not good as KOH from an economic and environmental point of view. On the other hand, desired product almost could not be observed with Et3N or DBU (entries 11 and 12). Screening of a range of solvents showed that the reaction did not proceed in DMF or DMSO (entries 13 and 14). Moderate to good yields were recorded in dioxane, PhCl and xylene, but the best result was obtained in toluene (entries 2 and 15–17). In addition, the reaction temperature was optimized. When the reaction temperature was decreased from 80 ℃ to 70 ℃, the yield of 3a was dramatically dropped to 41% (entry 18). However, the yield remained almost unchanged by increasing the temperature to 90 ℃ (entry 19). Further optimization showed that the optimum amounts of ABNO and KOH were 3 mol% and 30 mol%, respectively (entries 2, 20 and 21).
With the optimized conditions in hand, the scope and limitations of the present method were examined (Table 2). Firstly, 1a was chosen to react with a series of anilines bearing electron-donationing or electron-withdrawing substituents on ring under the optimized reaction conditions. It was observed that the presence of a substituent with different electronic properties on benzene ring of anilines had no significant influence on the reaction. All of the anilines could react with 1a smoothly to give the desired imine products in excellent yields (3a–3h). Moreover, it was found that the reaction was sensitive to the steric hindrance of the substituents on the phenyl ring, and meta- and para-substituted anilines exhibited higher activities than ortho-substituted isomer (3b–3d).
The scope of this catalytic method was further explored, and various substituted benzyl alcohols such as 4-chlorobenzyl alcohol, 4-methylbenzyl alcohol, 4-methoxybenzyl alcohol and 4-methylthiobenzyl alcohol were subjected to the oxidative coupling with 2a. It was found that imine products 3i–3l could be isolated in 92%–95% yields. Similarly, substituted benzyl alcohols could react smoothly with different anilines and afforded their corresponding substituted imine derivatives 3m–3r in good to excellent yields (80%–96%).
In addition, the representative polycyclic amine substrate, 1-naphthylamine tolerated this catalytic system, which oxidative coupled with 1a and 4-methoxybenzyl alcohol afforded 3s and 3t in 90% and 88% yields, respectively. What's more, 2-thiophenemethanol, a heteroatom-containing benzyl alcohol, successfully reacted with aniline and 4-methoxyaniline to give the corresponding imines 3u and 3v in excellent yields. To our delight, the reactions of aliphatic amines such as butan-1-amine and cyclopentanamine with different benzyl alcohols gave 3w–3z in 90%–91% yields. The reactions of aliphatic alcohols or 1-phenylethanol with aniline were also tested under the same conditions. Unfortunately, no desired imine products were detected.
|
|
Table 1 Optimization of reaction conditions.a |
|
|
Table 2 ABNO/KOH-catalyzed aerobic oxidative coupling of alcohols with amines to imines.a |
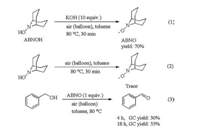
To gain some insight into the possible reaction mechanism, several control experiments were carried out. According to our recently report, KOH could accelerate the regeneration of ABNO from ABNOH under O2 atmosphere [17]. When ABNOH was reacted with KOH under air for 0.5 h, 70% yield of ABNO was isolated (Scheme 1, (1)). However, trace amount of ABNO was obtained when the reaction was carried out in the absence of KOH (Scheme 1, (2)). In addition, it was found that benzyl alcohol (1a) could be converted to benzaldehyde with 55% GC yield in 18 h in the presence of 1 equiv. of ABNO under air (Scheme 1, (3)).
|
Download:
|
| Scheme 1. Control experiments for mechanism studies. | |
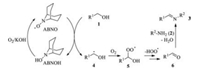
Based on the above observation and relevant literatures, we propose a probable mechanism for the formation of imines (Scheme 2). Initially, ABNO abstracts the hydrogen atom from benzyl alcohol 1 to form the corresponding α-hydroxyl carbon radical 4, and ABNO is converted to ABNOH. ABNOH can be reoxidized into ABNO by air in the presence of KOH. The radical 4 subsequently reacts with dioxygen to give the α-hydroxyl peroxyl radical 5, from which the carbonyl product 6 is formed by release of hydroperoxyl radical [18, 19]. The condensation of the amine 2 with the aldehyde 6 affords the product imine 3. In addition, this condensation reaction can promote the transformation of 1 to 6.
|
Download:
|
| Scheme 2. Plausible reaction pathways. | |
In summary, we have developed a simple and efficient method for preparation of imines by the oxidative coupling of alcohols and amines. The reaction was catalyzed by ABNO/KOH with air as the economic and green oxidant. Under the optimal reaction conditions, a wide range of products were synthesized in good to excellent yields.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21376224), the Natural Science Foundation of Zhejiang Province (No. LY17B060007), and Hangzhou Qianjiang Distinguished Experts Project.
| [1] |
(a) R. Bloch, Chem. Rev. 98 (1998) 1407-1438; (b) S. Kobayashi, H. Ishitani, Chem. Rev. 99 (1999) 1069-1094; (c) J. P. Adams, J. Chem. Soc. Perkin Trans. I 2 (2000) 125-139; (d) L. Yet, Angew Chem. Int. Ed. 40 (2001) 875-877; (e) D. Chen, Y. Wang, J. Klankermayer, Angew. Chem. Int. Ed. 49 (2010) 9475-9478; (f) J. Wang, X. Liu, X. Feng, Chem. Rev. 111 (2011) 6947-6983; (g) Y. Kobayashi, J. S. Mori, M. M. Fossey, Chem. Rev. 111 (2011) 2626-2704; (h) J. C. Adrio, Chem. Commun. 47 (2011) 6784-6794; (i) R. He, X. Jin, H. Chen, et al., J. Am. Chem. Soc. 136 (2014) 6558-6561; (j) M. Kondo, T. Kobayashi, N. Hatanaka, Y. Funahashi, S. Nakamura, Chem. -Eur. J. 1 (2015) 9066-9070; (k) B. Rohokale, D. D. Koenig, J. Org. Chem. 81 (2016) 7121-7126. |
| [2] |
(a) S. I. Murahashi, Angew. Chem. Int. Ed. Engl. 34 (1995) 2443-2465; (b) S. Yao, S. Saaby, R. G. Hazell, K. A. Jørgensen, Chem. -Eur. J. 6 (2000) 2435-2448; (c) P. Vicini, A. Geronikaki, M. Incerti, et al., Bioorg. Med. Chem. 11 (2003) 4785-4789; (d) Z. Y. Liu, Y. M. Wang, Z. R. Li, J. D. Jiang, D. W. Boykin, Bioorg. Med. Chem. Lett. 19 (2009) 5661-5664. |
| [3] |
(a) H. Schiff, Justus Liebigs Ann. Chem. 131 (1864) 118-119; (b) F. H. Westheimer, K. Taguchi, J. Org. Chem. 36 (1971) 1570-1572; (c) R. S. Varma, R. Dahiya, S. Kumar, Tetrahedron Lett. 38 (1997) 2039-2042; (d) G. Liu, D. A. Cogan, T. D. Owens, T. P. Tang, J. A. Ellman, J. Org. Chem. 64 (1999) 1278-1284; (e) M. A. Sprung, Chem. Rev. 26 (2002) 297-338; (f) H. Naeimi, F. Salimi, K. Rabiei, J. Mol. Catal. 260 (2006) 100-104; (g) J. T. Reeves, M. D. Visco, M. A. Marsini, et al., Org. Lett. 17 (2015) 2442-2445. |
| [4] |
(a) Q. Kang, Y. Zhang, Green Chem. 14 (2012) 1016-1019; (b) S. Musa, S. Fronton, L. Vaccaro, D. Gelman, Organometallics 32 (2013) 3069-3073; (c) D. Wang, X. Guo, C. Wang, et al., Adv. Syn. Catal. 355 (2013) 1117-1125; (d) J. F. Soule, H. Miyamura, S. Kobayashi, Chem. Commun. 49 (2013) 355-357; (e) K. T. Venkateswara Rao, B. Haribabu, P. S. Sai Prasad, N. Lingaiah, Green Chem. 15 (2013) 837-846; (f) J. Wang, S. Lu, X. Cao, H. Gu, Chem. Commun. 50 (2014) 5637-5640; (g) B. Chen, J. Li, W. Dai, L. Wang, S. Gao, Green Chem. 16 (2014) 3328-3334; (h) Z. Zhang, F. Wang, M. Wang, et al., Green Chem. 16 (2014) 2523-2527; (i) L. Wang, B. Chen, L. Ren, et al., Chin. J. Catal. 36 (2015) 19-23; (j) M. Tamura, K. Tomishige, Angew. Chem. In. Ed. 54 (2015) 864-867; (k) Z. Zhang, Y. Wang, M. Wang, et al., Chin. J. Catal. 36 (2015) 1623-1630; (l) S. Furukawa, A. Suga, T. Komatsu, ACS Catal. 5 (2015) 1214-1222; (m) W. Deng, J. Chen, J. Kang, Q. Zhang, Y. Wang, Chem. Commun. 52 (2016) 6805-6808; (n) R. R. Donthiri, R. D. Patil, S. Adimurthy, Eur. J. Org. Chem. 2012 (2012) 4457-4460. |
| [5] |
(a) L. Liu, S. Zhang, X. Fu, C. H. Yan, Chem. Commun. 47 (2011) 10148-10150; (b) H. Huang, J. Huang, Y. Liu, et al., Green Chem. 14 (2012) 930-934; (c) C. Su, M. Acik, K. Takai, et al., Nat. Commun. 3 (2012) 1298-1306; (d) L. Liu, Z. Wang, X. Fu, C. H. Yan, Org. Lett. 14 (2012) 5692-5695; (e) X. H. Li, M. Antonietti, Angew. Chem. Int. Ed. 52 (2013) 4572-4576; (f) A. Monopoli, P. Cotugno, F. Iannone, et al., Eur. J. Org. Chem. 2014 (2014) 5925-5931; (g) H. Wang, X. Zheng, H. Chen, et al., Chem. Comm. 50 (2014) 7517-7520; (h) B. Chen, L. Wang, W. Dai, et al., ACS Catal. 5 (2015) 2788-2794. |
| [6] |
(a) J. H. Park, K. C. Ko, E. Kim, et al., Org. Lett. 14 (2012) 5502-5505; (b) N. Li, X. Lang, W. Ma, et al., Chem. Commun. 49 (2013) 5034-5036; (c) S. Naya, K. Kimura, H. Tada, ACS Catal. 3 (2013) 10-13; (d) W. Zhao, C. Liu, L. Cao, et al., RSC Adv. 3 (2013) 22944-22948; (e) X. Lang, X. Chen, J. Zhao, Chem. Soc. Rev. 43 (2014) 473-486; (f) X. Lang, W. Ma, C. Chen, H. Ji, J. Zhao, J. Acc. Chem. Res. 47 (2014) 355-363; (g) X. J. Yang, B. Chen, X. B. Li, et al., Chem. Commun. 50 (2014) 6664-6667; (h) B. Yuan, R. Chong, B. Zhang, et al., Chem. Commun. 50 (2014) 14493-14496; (i) D. Sun, L. Ye, Z. Li, Appl. Catal. B: Environ. 164 (2015) 428-432. |
| [7] |
(a) M. Largeron, A. Chiaroni, M. B. Fleury, Chem. -Eur. J. 14 (2008) 996-1003; (b) A. E. Wendlandt, S. S. Stahl, Org. Lett. 14 (2012) 2850-2853; (c) M. Largeron, M. B. Fleury, Angew. Chem. Int. Ed. 124 (2012) 5505-5508; (d) H. Yuan, W. J. Yoo, H. Miyamura, S. Kobayashi, J. Am. Chem. Soc. 134 (2012) 13970-13973; (e) M. Largeron, M. B. Fleury, Science 339 (2013) 43-44. |
| [8] |
B. Chen, L. Wang, S. Gao, ACS Catal. 5 (2015) 5851-5876. DOI:10.1021/acscatal.5b01479 |
| [9] |
H. Tian, X. Yu, Q. Li, J. Wang, Q. Xu, Adv. Synth. Catal. 354 (2012) 2671-2677. DOI:10.1002/adsc.v354.14/15 |
| [10] |
M. Guan, C. Wang, J. Zhang, Y. Zhao, RSC Adv. 4 (2014) 48777-48782. DOI:10.1039/C4RA09851J |
| [11] |
L. Jiang, L. Jin, H. Tian, et al., Chem. Commun. 47 (2011) 10833-10835. DOI:10.1039/c1cc14242a |
| [12] |
E. Zhang, H. Tian, S. Xu, X. Yu, Q. Xu, Org. Lett. 15 (2013) 2704-2707. DOI:10.1021/ol4010118 |
| [13] |
(a) Y. Xie, W. Mo, D. Xu, et al., J. Org. Chem. 72 (2007) 4288-4291; (b) X. He, Z. Shen, W. Mo, et al., Adv. Synth. Catal. 351 (2009) 89-92; (c) Q. Chen, C. Fang, Z. Shen, M. Li, Electrochem. Commun. 64 (2016) 51-55; (d) C. Fang, M. Li, X. Hu, et al., Adv. Synth. Catal. 358 (2016) 1157-1163; (e) J. Ma, C. Hong, Y. Wan, et al., Tetrahedron Lett. 58 (2017) 652-657; (f) X. Yang, Z. Fan, Z. Shen, M. Li, Electrochim. Acta 226 (2017) 53-59. |
| [14] |
M. Shibuya, M. Tomizawa, Y. Sasano, Y. Iwabuchi, J. Org. Chem. 74 (2009) 4619-4622. DOI:10.1021/jo900486w |
| [15] |
T.R. Porter, D. Capitao, W. Kaminsky, Z. Qian, J.M. Mayer, Inorg. Chem. 55 (2016) 5467-5475. DOI:10.1021/acs.inorgchem.6b00491 |
| [16] |
J. Xu, R. Zhuang, L. Bao, G. Tang, Y. Zhao, Green Chem. 14 (2012) 2384-2387. DOI:10.1039/c2gc35714c |
| [17] |
J. Ma, Y. Wan, C. Hong, et al., Eur. J. Org. Chem. 23 (2017) 3335-3342. |
| [18] |
C. Annunziatini, M.F. Gerini, O. Lanzalunga, M. Lucarini, J. Org. Chem. 69 (2004) 3431-3438. DOI:10.1021/jo049887y |
| [19] |
Y. Hu, L. Chen, B. Li, Catal. Commun. 83 (2016) 482-487. |
 2018, Vol. 29
2018, Vol. 29