b College of Pharmacy, Jinan University, Guangzhou 510632, China;
c School of Life Sciences, Sun Yat-sen University, Guangzhou 510275, China;
d School of Pharmaceutical Science, Sun Yat-sen University, Guangzhou 510006, China;
e State Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources, South China Agricultural University, Guangzhou 510642, China
Neem (Azadirachta indica A. Juss) is a plant belonging to the meliaceae family and originally distributed India and Burma [1, 2]. Neem has been used as a traditional medicine plant in India for over thousands of years, for treating cholera, diarrheal, skin ulcers, intestinal worms, diabetes, leprosy, obstinate urinary disorder, etc. [1, 3, 4]. More than 300 compounds were isolated from Neem, and over 130 of them belong to limonoid-type triterpenoids [5].
Limonoids are a group of tetranortriterpenoids losing four terminal carbons of the side chain in the apotirucallane or apoeuphane backbones, and in the most cases the rest carbons of their side chain form a furan ring [5]. Limonoids possess a variety of activities, including anti-inflammation [6, 7], antimalarial [8] and antibacterial [9], etc. Recently, cytotoxic activity of limonoidtype triterpenoids was extensively studied. Notably, it was reported that two main limonoids nimbolide and azadirone showed remarkable effects on inhibiting growth of cancer cell lines. Nimbolide can inhibit pancreatic cancer growth through induction of ROS-mediated apoptosis [10], and hamster buccal pouch carcinomas by downregulating P13 K/Akt signaling with consequent activation of glycogen synthase kinase-3β [11]. Azadirone can enhance the efficacy of tumor necrosis factorrelated apoptosis-inducing ligand against cancer cells [12]. However, all the works only focused on nimbolide and azadirone, two main limonoid metabolites of neem, while other minor limonoids were little studied. Therefore, it is necessary to conduct a further study on limonoid-type derivatives and their anticancer activity. In the present study, a new limonoid alkaloid, azadiramide A (1) (Fig. 1), was isolated from seeds of A. indica A. Juss, and exhibited inhibitory activity against human breast cancer MDAMB-231 cell line.
|
Download:
|
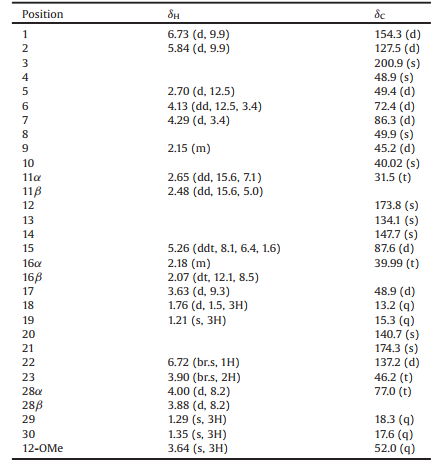
| Fig. 1. The structure of compound 1. | |
Compound 1 was obtained as a white amorphous powder. Its HRESIMS displayed the quasi-molecular ion peaks at m/z 468.2394 [M+H]+ (calcd. 468.2386) and 935.4706 [2M+H]+ (calcd. 935.4694), indicating its molecular formula is C27H33NO6. The 1H NMR and 13C NMR spectra of 1 (Table 1) implied the presence of an α, β-unsaturated keto [δH 6.73 (d, J = 9.9 Hz, H-1) and 5.84 (d, J = 9.9 Hz, H-2); δC 200.9 (C-3), 154.3 (C-1) and 127.5 (C-2)]. Four sp2 carbon signals (δC 134.1, 147.7, 137.2 and 140.7) attributed to two double bonds in the 13C NMR spectrum, together with an olefinic proton [δH 6.72 (br.s, H-22)] in the 1H NMR spectrum, indicated they were tri-substituted and tetra-substituted, respectively.
|
|
Table 1 1H (600 MHz) and 13C NMR (151 MHz) data of compound 1 (recorded in CDCl3; δ in ppm, J in Hz). |
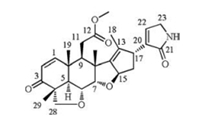
The structure of 1 was finally constructed by its 2D NMR spectra. The proton and protonated carbon resonances in the NMR spectra of 1 were firstly assigned by the HSQC experiment. The 1H-1H COSY correlations H-1/H-2, H-5/H-6/H-7, H-9/H2-11 and H-15/H2-16/H-17 indicated four structural fragments labelled in red in Fig. 2. In the HMBC spectrum, the correlations from H-1 to C-3, from H3-29 to C-3, C-4, C-5 and C-28, from H3-19 to C-1, C-5, C-9 and C-10, from H3-30 to C-7, C-8, C-9 and C-14, and from H3-18 to C-13, C-14 and C-17 tethered four red fragments together with the blue ones shown in Fig. 2. The HMBC correlation of H-9/C-12, together with the chemical shift of C-12, indicated that C-12 was linked to C-11 and oxidized into a carboxyl group which was methylated due to the HMBC correlation between H12-OMe and C-12. Two tetrahydrofuran rings were constructed by the correlations between H-28 and C-6, and between H-7 and C-15 in the HMBC spectrum. The above structural fragments implied that 1 could be a salannin-class limonoid belonging to ring C-seco group [5]. Thus, the unassigned fragment was C4H4NO, including a carbonyl carbon (C-21, δC 174.3), a trisubstituted double bond [C-20 and C-22, δC 140.7 and 137.2; H-22, δH 6.72 (br.s, 1H)], an isolated methylene group [C-23, δC 46.2; H2-23, δH 3.90 (br.s, 2H)] and a NH, which were a group of characteristic signals attributed to a 3-substituted 1, 5-dihydro-2H-pyrrol-2-one unit [13]. This was also confirmed by the HMBC correlations from H-22 to C-17, C-20, C-21 and C-23, and from H2-23 to C-20, C-21 and C-22. Therefore, the planar structure of 1 was elucidated and was a rare salannin-class limonoid alkaloid. To our best knowledge, only two compounds belonging to this type were so far found [13].
|
Download:
|
| Fig. 2. Selective 2D NMR correlations of compound 1. | |
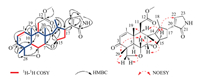
The relative configuration of 1 was determined by its NOESY spectrum. The NOE correlations from H-6 to H3-19, H3-29 and H3-30, from H3-30 to H-7 and H2-11, from H2-11 to H3-19 and from H3-29 to H-28β indicated these protons were oriented on the same side of the ring system and arbitrarily assigned as β. On the other hand, the NOE correlations from H-5 to H-28α, from H-15 to H-9, H-16a and H-22, and from H-16α to H-22 assigned H-5, H-9, H-15 and the lactam ring to α configuration. The absolute stereochemistry of 1 was determined according to comparison of its experimental CD spectrum with the calculated ones [14], as shown in Fig. 3. Three lowest energy conformers of 1 were given in Fig. S1 (Supporting information). The experimental CD spectrum showed two positive CEs around 205 nm and 240 nm which excellently matched its calculated spectrum and was opposite to the spectrum of its enantiomer.
|
Download:
|
| Fig. 3. Experimental CD spectrum of compound 1 (black) overlaid with the calculated ECD spectra of 1 (red) and its enantiomer (blue). | |
In a cytotoxic assay against human lung carcinoma A549, breast cancer MDA-MB-231 and colon carcinoma HCT-116 cell lines, 1 showed inhibitory activity against human breast cancer MDA-MB-231 cell line with IC50 value of 2.70 ± 0.63 μmol/L. The positive control cisplatin inhibited growth of A549, MDA-MB-231 and HCT-116 cells with IC50 of 7.06 ± 2.15, 1.70 ± 0.43 and 3.94 ± 0.67 μmol/L, respectively.
In summary, a rare salannin-class limonoid alkaloid, azadiramide A (1), was isolated from seeds of A. indica A. Juss. So far, only 27 limonoid alkaloids were found in nature, including 2 ring intact [15], 10 ring D-seco [15], 13 ring A/B-seco [16, 17] and 2 ring C-seco (salannin-class) limonoid alkaloids [13]. These compounds possess lactams as their side chains. Ring intact limonoid alkaloids possess a 6/6/6/5 ring system which is same as those of normal tetracyclic triterpenoids [15]. In ring D-seco limonoid alkaloids, oxidative cleavage occurs at the bond between C-16 and C-17, and an ester bond subsequently forms between them [15]. In ring A/B-seco limonoid alkaloids, both bonds of C-3/C-4 and C-7/C-8 are cleaved by oxidation reactions, and two types of formation of lactone rings could occur, in the first type of which an ester bond forms between C-3 and C-4 [17] and in the second type of which two ester bonds form between C-3 and C-11, and between C-7 and C-4 [16]. In ring C-seco limonoid alkaloids, ring C breaks and C-12 is oxidized into carboxyl group which is subsequently methylated, on the other hand, two tetrahydrofuran rings form via two ether bonds of C-28/O/C-6 and C-7/O/C-15 [13]. Therefore, 1 is the third example of ring C-seco limonoid alkaloids and can inhibit growth of human breast cancer MDA-MB-231 cell line with IC50 value of 2.70± 0.63μmol/L.
AcknowledgmentThis work was supported by grants from Thousand Young Talents Program of China and the National Natural Science Foundation of China (No. 81673530).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.11.042.
| [1] |
V.S. Kumar, V. Navaratnam, Asian Pac. J. Trop. Biomed. 3 (2013) 505-514. DOI:10.1016/S2221-1691(13)60105-7 |
| [2] |
S. Senthil-Nathan, Front. Physiol. 4 (2013) 359. |
| [3] |
P. Thakurta, P. Bhowmik, S. Mukherjee, et al., J. Ethnopharmacol. 111 (2007) 607-612. DOI:10.1016/j.jep.2007.01.022 |
| [4] |
G. Brahmachari, ChemBioChem 5 (2004) 408-421. DOI:10.1002/cbic.v5:4 |
| [5] |
Q.G. Tan, X.D. Lou, Chem. Rev. 111 (2011) 7437-7522. DOI:10.1021/cr9004023 |
| [6] |
T. Akihisa, T. Noto, A. Takahashi, et al., J. Oleo Sci. 58 (2009) 581-594. DOI:10.5650/jos.58.581 |
| [7] |
A. Alam, S. Haldar, H.V. Thulasiram, et al., J. Biol. Chem. 287 (2012) 24844-24861. DOI:10.1074/jbc.M112.341321 |
| [8] |
S.A. Khalid, H. Duddeck, M. Gonzalez-Sierra, J. Nat. Prod. 52 (1989) 922-927. DOI:10.1021/np50065a002 |
| [9] |
W. Maneerata, S. Laphookhieoa, S. Koysomboonb, et al., Phytomedicine 15 (2008) 1130-1134. DOI:10.1016/j.phymed.2008.05.004 |
| [10] |
R. Subramani, E. Gonzalez, A. Arumugam, et al., Sci. Rep. 6 (2016) 19819. DOI:10.1038/srep19819 |
| [11] |
J. Sophia, T.K. Kiran Kishore, J. Kowshik, et al., Sci. Rep. 6 (2016) 22192. DOI:10.1038/srep22192 |
| [12] |
S.C. Gupta, S.K. Francis, M.S. Nair, et al., J. Biol. Chem. 288 (2013) 32343-32356. DOI:10.1074/jbc.M113.455188 |
| [13] |
W. Kraus, A. Klenk, M. Bokel, et al., Liebigs Ann. Chem. (1987) 337-340. |
| [14] |
L.J. Meng, Q.L. Guo, C.G. Zhu, et al., Chin. Chem. Lett. 29 (2018) 119-122. DOI:10.1016/j.cclet.2017.05.019 |
| [15] |
J.H. Li, Y. Li, F.L. An, et al., Tetrahedron 72 (2016) 7481-7487. DOI:10.1016/j.tet.2016.09.061 |
| [16] |
S.H. Qi, L. Chen, D.G. Wu, et al., Tetrahedron 59 (2003) 4193-4199. DOI:10.1016/S0040-4020(03)00573-8 |
| [17] |
G.Y. Zhu, G. Chen, L. Liu, et al., J. Nat. Prod. 77 (2014) 983-989. DOI:10.1021/np401089h |
 2018, Vol. 29
2018, Vol. 29