The dried roots of Isatis indigotica Fort. (Cruciferae), named "ban lan gen" in Chinese, is among the most popular herbal drugs for the treatment of colds, especially of the flu during influenza pandemics in China [1]. Many formulations containing extracts of "ban lan gen" or the raw material are compiled in Chinese Pharmacopoeia [2]. Agricultural cultivation of this plant not only fulfils demands of medicinal industry but also plays roles of environment protection and increasing local economy in several provinces of China. Considerable investigations on this herbal medicine indicated that "ban lan gen" extracts had antiviral, anti-endotoxic, anti-inflammatory, antipyretic, and cytotoxic activities [3-7] and contained alkaloids [8-13], lignans [14, 15], flavonoids [16], and epigoitrin and related analogues [17]. However, the previous chemical studies were mainly carried out on the ethanol or methanol extracts of the drug material, which differs from a practical application by decocting with water. Therefore, an aqueous decoction of "ban lan gen" was investigated as part of a program to systematically study the chemical diversity of traditional Chinese medicines and their biological activities [18-39]. More than 100 compounds including 61 with new structures from the decoction were reported in our previous papers [39-51]. Antiviral activity against influenza virus A/Hanfang/359/95 (H3N2) or Coxsackie virus B3, protective activity against dl-galactosamine-induced hepatocyte (WB-F344 cell) damage, and inhibitory activity against the LPS-induced NO production in mouse peritoneal macrophage [40, 41, 43-45, 47] were found for the isolates with varied structural types. A continuation of the study, focusing on the minor constituents, has led to characterization of an unusual glucosidic indole-lignan conjugate with a novel carbon skeleton, named isatindolignanoside A (1) (Fig. 1). This paper describes details of the isolation, structure elucidation, and bioassay of the new isolate.

|
Download:
|
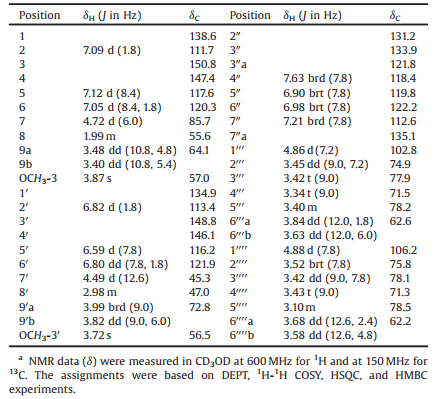
| Fig. 1. Structure of compound 1. | |
Compound 1 was isolated as a yellowish amorphous powder with [α]D20 -28.3 (c 0.04, MeOH). Its IR spectrum displayed absorption bands due to hydroxyl (3395 cm-1) and aromatic (1600 cm-1 and 1515 cm-1) functionalities. The ESIMS of 1 exhibited quasimolecular ion peaks at m/z 838 [M+Na]+ and 814 [M-H]-. The molecular formula C40H49NO17 was determined by HR-ESIMS at m/z 838.2905 [M+Na]+ (calcd. for C40H49NO17Na: 838.2893), in combination with the NMR data (Table 1). The 1H NMR spectrum of 1 in CD3OD showed resonances assignable to (a) two meta-para-disubstituted phenyls at δH 7.09 (d, J = 1.8 Hz, H-2), 7.12 (d, J = 8.4 Hz, H-5), and 7.05 (dd, J = 8.4 and 1.8 Hz, H-6) and 6.82 (d, J = 1.8 Hz, H-2'), 6.59 (d, J = 7.8 Hz, H-5'), and 6.80 (dd, J = 7.8 and 1.8 Hz, H-6'); (b) an ortho-substituted phenyl at δH 7.63 (brd, J = 7.8 Hz, H-4''), 6.90 (brt, J = 7.8 Hz, H-5''), 6.98 (brt, J = 7.8 Hz, H-6''), and 7.21 (brd, J = 7.8 Hz, H-7''); and (c) two methoxy groups at δH 3.87 (s, 3-OCH3) and 3.72 (s, 30-OCH3). In addition, the spectrum showed resonances assignable to two β-glucopyranosyls, two oxygen-bearing methylenes, and four methines (one oxygenbearing) between δH 4.89 and 1.99. The β-configuration of the glucopyranosyls were indicated by the coupling constant value of the anomeric protons resonated at δH 4.86 (d, J = 7.2 Hz, H-1''') and 4.88 (d, J = 7.8 Hz, H-1''''), respectively. The 13C NMR and DEPT spectra of 1 showed 40 carbon signals corresponding to the abovedescribed functional units, as well as two additional sp2 hybridized quaternary carbons attributed to a tetra-substituted double bond. As compared with those of compounds previously isolated from I. indigotica [40-51], these spectroscopic data suggest that 1 is an unusual alkaloid diglucopyranoside, of which the structure was further elucidated by 2D NMR data analysis.
|
|
Table 1 NMR spectroscopic data for compound 1.a |
The proton-bearing carbon resonances and corresponding proton resonances in the NMR spectra were readily assigned by HSQC spectroscopic data analysis (Table 1). The presence of the three phenyls were confirmed by cross-peaks of the ortho- and meta-coupling aromatic protons H-5/H-6/H-2, H-5'/H-6'/H-2', and H-4''/H-5''/H-6''/H-7'' (Fig. 2, thick lines) in the 1H-1H COSY spectrum of 1. Meanwhile, vicinal coupling correlations of H-1'''/H-2'''/H-3'''/H-4'''/H-5'''/H2-6''' and H-1''''/H-2''''/H-3''''/H-4''''/H-5''''/H2-6'''' proved the presence of the two β-glucopyranosyls. Additionally, the 1H-1H COSY spectrum showed the correlations of H-7/H-8/H2-9 and H-7'/H-8'/H2-9', indicating that there were 7, 7, 8- and 7', 7', 8'-trisubstituted propyloxy units in 1. In the HMBC spectrum of 1, two- and three-bond heteronuclear correlations (Fig. 2, arrows) from H-2 and H-6 to C-4 and C-7; from H-5 to C-1 and C-3; from 3-OCH3 to C-3; from H-7 to C-1, C-2 and C-6; and from H-1''' to C-4; together with the chemical shifts of these proton and carbon resonances; indicated that C-7 of one propyloxy unit was substituted by a 4-β-glucopyranosyloxy-3-methoxyphenyl. Similarly substitution at C-7' of the other propyloxy unit by a 4'-oxy-3'-methoxyphenyl was evidenced by the correlations from H-2' and H-6' to C-4' and C-7'; from H-5' to C-1' and C-3'; from 3'-OCH3 to C-3'; and from H-7' to C-1', C-2', and C-6', along with their chemical shift values. The HMBC correlations from H-7 to C-8' and from both H-7' and H2-9' to C-8 revealed that the two phenylpropyloxy units connected via the C—8—C—8' bond to give a lignan moiety in 1. The HMBC correlations of H-7/C-9' and H2-9'/C-7, in combination with the chemical shift values of C-7 and C-9', indicated an oxygen-bridged linkage between C-7 and C-9' to form a tetrhydrofuran ring. Meanwhile, the HMBC spectrum of 1 showed correlations from H-4'' to C-3'', C-3''a, C-6'', and C-7''a; from H-5'' to C-3''a, C-6'', and C-7''; from H-6'' to C-4'' and C-7''a; from H-7'' to C-3''a and C-5''; and from H-1'''' to C-3''. These correlations, combined with their chemical shift values, demonstrated that one end (C-3'') of tetra-substituted double bond not only connected to C-3''a of the ortho-substituted phenyl but also was substituted by the remaining β-glucopyranosyloxy. In addition, the HMBC correlations of both C-2'' and C-3'' with H-7' established a connection between C-2'' and C-7'. Taking into account of unconnected sites C-2'', C-7''a, C-4', and C-9, especially the chemical shift values of these carbon resonances, as well as the molecular formula, the two carbons C-2'' and C-7''a must connected via the remaining nitrogen atom to construct an indole ring while the carbons C-4' and C-9 must be substituted by two hydroxyls. Therefore, the planar structure of 1 was deduced to be an unusual lignan-coupled indole alkaloid diglucopyranoside as shown in Fig. 2. The deduction was supported by comparing the NMR spectroscopic data of 1 with those of the co-occurring lignanoid and indole derivatives [42].

|
Download:
|
| Fig. 2. The 1H-1H COSY (thick lines) and three-bond HMBC correlations (red arrows, from 1H to 13C) of 1. | |
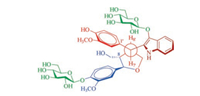
The configuration of 1 was determined by J-based configurational analysis and NOESY spectrum, together with enzyme hydrolysis and circular dichroism (CD) data. In the 1H NMR spectrum, the large 3JH-7', H-8' coupling constant (12.6 Hz) revealed that free rotation of the single bond between C-7' and C-8' was restricted in solution and that the two vicinal protons H-7' and H-8' were anti-oriented in a stabilized conformation of 1 (Figs. 3 and 4). In the NOESY spectrum, correlations between H-7' with H-6 and H-8, indicated that these protons were cofacial on one side of the tetrahydro-furan ring (Fig. 3). The NOESY correlations between H-8' and H2-9 demonstrated that these protons were oriented on the other side of the tetrahydrofuran ring. Especially the NOESY correlations between both H-2' and H-6' with H-8 indicated that the C—1'-C—7' and C—8—C—8' bonds were gauche-oriented as shown in the Newman projection viewing from C—7' to C—8' (Fig. 4). Thus, the relative configuration of 1 was established, which was consistent with the lowest-energy 3D conformation obtained by Monte Carlo searching with the MMFF94 molecular mechanics force field using the MOE software [52] (Fig. 3).

|
Download:
|
| Fig. 3. The NOESY correlations (pink dashed double arrows) of 1. | |
|
Download:
|
| Fig. 4. Newman projection of a conformation visualizing from C-7' to C-8' based on 3JH-7', H-8' and NOESY correlation analysis of 1. | |
Enzymatic hydrolysis of 1 liberated D-glucose, which was isolated from the hydrolysate and identified by comparison of TLC and the 1H NMR spectroscopic and specific rotation data with those of an authentic D-glucose sample, while the aglycone was decomposed into a complex mixture which failed to be separated due to limitation of the sample amount (Figs. S26 and S27 in Supporting information). The circular dichroism (CD) spectrum of 1 showed a negative Cotton effect at λmax 237.5 (Δe -24.09) and a positive Cotton effect at λmax 293.0 (Δε +6.66) nm. As compared with the CD data of the lignanoids and indole derivatives isolated from the same extract [40-51], the Cotton effects arose from the π→π* transitions of three chromophores in 1. Because of transition overlapping of the chromophores and because of the absence of characteristic exciton coupling Cotton effects, the available CD rules may not be applicable for determination of the absolute configuration. Meanwhile, our efforts to crystallize 1 in a variety of solvent systems failed to determine the absolute configuration by single crystal X-ray diffraction analysis. Subsequently, the theoretical electronic CD (ECD) spectra of 1, its diastereoisomer (1'), and the enantiomeric aglycones (1a and 1'a) were calculated by using a method based on quantum mechanical time dependent density functional theory (TDDFT) [53]. Extensive comparison of the calculated ECD spectra of 1, 1', 1a, and 1'a revealed that the β-glucopyranosyls on the chromophores of 1 and 1' dominated intensities, wavelengths, and signs of the Cotton effects in a long wavelength region (> 250 nm) of the calculated ECD spectra (Supporting information). However, in a low wavelength region (200–245 nm), the Cotton effect curves of 1 and 1' were distinguishable. As compared, in the distinctive region the experimental CD curve is in well agreement with the calculated curve of 1 but obviously different from that of 1', though in the long wavelength region (> 250 nm) the two theoretical Cotton effects are replaced by only one relative weak Cotton effect in the experimental CD spectrum (Fig. 5). This suggests the 7R, 7'R, 8S, 8'R configuration for 1. Therefore, the structure of compound 1 was determined and named isatindolignanoside A, which has the systematical name (–)-(7R, 7'R, 8S, 8'R)-4-β-D-glucopyranosyloxy-3, 3'-dimethoxy-7'-(3''-β-D-glucopyranosyloxy-1''H-indol-2''-yl)-7, 9'-epoxylignane-4', 9-diol according to IUPAC commented nomenclature of lignans and neolignans [54].

|
Download:
|
| Fig. 5. The overlaid experimental CD (black) and calculated ECD spectra of 1 (red dash) and 1' (blue dot). | |
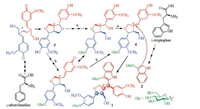
Isatindolignanoside A (1) possesses a novel skeleton of indolelignan conjugation, which represents the first example of its kind. Based on the unique structural feature, the plausible biosynthetic pathway for 1 is postulated in Scheme 1. The biosynthetic precursor of 1 is proposed to be isatan B (2) and (-)-pinoresinol (3), since 2 and 3 as well as their derivatives abundantly co-occur in this plant [39-51], which are metabolites biosynthesized from L-phenylanaline (L-tryptophan) and L-tryptophan, respectively. Because the C-8' configuration in 1 was reversed comparing to corresponding chiral center in the precursor 3, a C-8'/C-7' conjugated intermediate 4 was proposed to be formed in the biosynthetic pathway. The intermediate 4 could be generated from 3 either via (a) an enzyme-catalyzed sequential hydrolysis and dehydration process or (b) an oxidative opening of one tetrahydrofuran ring followed by intramolecular double bond rearrangement. The intermediate 4 undergoes a nucleophilic addition with 2 to afford 1. Glucosidation may take place during these processes. In order to exclude possible formation of 1 during the isolation procedure, 2 and (–)-pinoresinol mono- or di-glucosides (5 or 6, Figs. S28 and S29 in Supporting information) was refluxed in H2O or methanol with or without silica gel (the main solvents and absorbent used in the isolation procedure) for 48 h. HPLC analysis of the reaction mixtures indicated that 1 was not formed under the simulated conditions. This supports that compound 1 is a natural product.
|
Download:
|
| Scheme 1. The plausible biosynthetic pathway of 1. | |
In the preliminary antiviral assay [40-51], compound 1 was active against Coxsackie virus B3 with IC50 and SI values of 25.9 ×10-6 mol/L and > 3.9, respectively, the positive controls pleconaril and ribavirin gave IC50 values of 0.001 × 10-6 mol/L and 517.4 × 10-6 mol/L and SI values of 17122.2 and 3.9, respectively. However, 1 was inactive against influenza virus A/Hanfang/359/95 (H3N2) at a concentration of 10-5 mol/L.
In conclusion, isatindolignanoside A (1) with antiviral activity against Coxsackie virus B3 was isolated as the minor constituent from the aqueous extract of "ban lan gen". This result, together with our previous studies [40-51], continuously proves that the multiple chemical constituents with the diverse structures have contributions towards clinical efficacy that supports the conventional applications of "ban lan gen". Particularly the structure of 1 possesses the novel carbon skeleton connecting between indole and lignan parent structures via a carbon-carbon bond, and represents the first indole-lignan conjugating natural product. The structural novelty not only enriches the diversity of the bioactive chemical constituents of "ban lan gen", but also challenges synthetic and biosynthetic chemists to obtain enough amount of the sample for further biological evaluation.
AcknowledgmentFinancial support from the National Natural Science Foundation of China (Nos. 81373287, 81630094, and 30825044) is acknowledged.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.12.001.
| [1] |
Jiangsu New Medical College, Dictionary of Traditional Chinese Medicine, Shanghai Science and Technology Publishing House, Shanghai, 1986.
|
| [2] |
Chinese Pharmacopoeia Commission, Pharmacopoeia of People's Republic of China. Part 1. Beijing: China Medical Science Press, 2015.
|
| [3] |
A.H. Lin, S.X. Fang, J.G. Fang, G. Du, Y.H. Liu, Chin. J. Chin. Mater. Med. 27 (2002) 439-442. |
| [4] |
J.G. Fang, J. Tang, Z.Q. Yang, et al., Chin. Tradit. Herb. Drugs 36 (2005) 242-244. |
| [5] |
L. Chen, T. Lin, H. Zhang, Y. Su, Intervirology 48 (2005) 207-212. DOI:10.1159/000084596 |
| [6] |
Y.L. Ho, Y.S. Chang, Phytomedicine 9 (2002) 419-424. DOI:10.1078/09447110260571661 |
| [7] |
S.L. Hsuan, S.C. Chang, S.Y. Wang, et al., J. Ethnopharm. 123 (2009) 61-67. DOI:10.1016/j.jep.2009.02.028 |
| [8] |
B. Li, W.S. Chen, S.Q. Zheng, G.J. Yang, C.Z. Qiao, Acta Pharm. Sin. 35 (2000) 508-510. |
| [9] |
W.S. Chen, B. Li, W.D. Zhang, G.J. Yang, C.Z. Qiao, Chin. Chem. Lett. 12 (2001) 501-502. |
| [10] |
X.Y. Wei, C.Y. Leung, C.K.C. Wong, et al., J. Nat. Prod. 68 (2005) 427-429. DOI:10.1021/np049662i |
| [11] |
J.F. Liu, Z.Y. Jiang, R.R. Wang, et al., Org. Lett. 9 (2007) 4127-4129. DOI:10.1021/ol701540y |
| [12] |
Y. Wu, Z.X. Zhang, H. Hu, et al., Fitoterapia 82 (2011) 288-292. DOI:10.1016/j.fitote.2010.10.016 |
| [13] |
L. Yang, G. Wang, M. Wang, et al., Fitoterapia 95 (2014) 175-181. DOI:10.1016/j.fitote.2014.03.019 |
| [14] |
L.W. He, X. Li, J.W. Chen, et al., Acta Pharm. Sin. 41 (2006) 1193-1196. |
| [15] |
L. Zuo, J.B. Li, J. Xu, et al., Chin. J. Chin. Mater. Med. 32 (2007) 688-691. |
| [16] |
Y. He, J. Lu, R.C. Lin, Chin. Tradit. Herb. Drugs 34 (2003) 777-778. |
| [17] |
Q.S. Huang, K. Yoshihira, S. Natori, Planta Med. 42 (1981) 308-310. DOI:10.1055/s-2007-971650 |
| [18] |
W.D. Xu, Y. Tian, Q.L. Guo, Y.C. Yang, J.G. Shi, Chin. Chem. Lett. 25 (2014) 1531-1534. DOI:10.1016/j.cclet.2014.09.012 |
| [19] |
Y. Tian, Q.L. Guo, W.D. Xu, et al., Org. Lett. 16 (2014) 3950-3953. DOI:10.1021/ol501760h |
| [20] |
W.X. Song, Y.C. Yang, J.G. Shi, Chin. Chem. Lett. 25 (2014) 1215-1219. DOI:10.1016/j.cclet.2014.05.037 |
| [21] |
Z.B. Jiang, W.X. Song, J.G. Shi, Chin. Chem. Lett. 26 (2015) 69-72. DOI:10.1016/j.cclet.2014.10.011 |
| [22] |
Y. Yu, Z. Jiang, W. Song, et al., Acta Pharm. Sin. B 5 (2015) 210-214. DOI:10.1016/j.apsb.2015.01.012 |
| [23] |
W.X. Song, Q.L. Guo, Y.C. Yang, J.G. Shi, Chin. Chem. Lett. 26 (2015) 517-521. DOI:10.1016/j.cclet.2014.11.035 |
| [24] |
Y. Jiang, Y. Liu, Q. Guo, et al., Acta Pharm. Sin. B 5 (2015) 215-222. DOI:10.1016/j.apsb.2015.03.005 |
| [25] |
Y.P. Jiang, Y.F. Liu, Q.L. Guo, et al., J. Asian Nat. Prod. Res. 17 (2015) 601-614. DOI:10.1080/10286020.2015.1041932 |
| [26] |
Y.P. Jiang, Y.F. Liu, Q.L. Guo, J.G. Shi, J. Asian Nat. Prod. Res. 17 (2015) 1166-1179. DOI:10.1080/10286020.2015.1112797 |
| [27] |
Y.P. Jiang, Q.L. Guo, Y.F. Liu, J.G. Shi, Chin. Chem. Lett. 27 (2016) 55-58. DOI:10.1016/j.cclet.2015.11.009 |
| [28] |
Y. Jiang, Y. Liu, Q. Guo, et al., Acta Pharm. Sin. B 6 (2016) 46-54. DOI:10.1016/j.apsb.2015.09.007 |
| [29] |
Z.B. Jiang, B.Y. Jiang, C.G. Zhu, et al., J. Asian Nat. Prod. Res. 16 (2014) 891-900. DOI:10.1080/10286020.2014.939585 |
| [30] |
Z.B. Jiang, X.H. Meng, B.Y. Jiang, et al., Chin. Chem. Lett. 26 (2015) 653-656. DOI:10.1016/j.cclet.2015.04.011 |
| [31] |
X.H. Meng, Z.B. Jiang, C.G. Zhu, et al., Chin. Chem. Lett. 27 (2016) 993-1003. DOI:10.1016/j.cclet.2016.05.013 |
| [32] |
X.H. Meng, Z.B. Jiang, Q.L. Guo, J.G. Shi, Chin. Chem. Lett. 28 (2017) 588-592. DOI:10.1016/j.cclet.2016.11.010 |
| [33] |
X.H. Meng, Q.L. Guo, C.G. Zhu, J.G. Shi, Chin. Chem. Lett. 28 (2017) 1705-1710. DOI:10.1016/j.cclet.2017.04.026 |
| [34] |
Q. Guo, Y. Wang, S. Lin, et al., Acta Pharm. Sin. B 5 (2015) 350-357. DOI:10.1016/j.apsb.2015.02.002 |
| [35] |
Q.L. Guo, Y.N. Wang, C.G. Zhu, et al., J. Asian Nat. Prod. Res. 17 (2015) 439-454. DOI:10.1080/10286020.2015.1040000 |
| [36] |
J. He, Z. Luo, L. Huang, et al., Anal. Chem. 87 (2015) 5372-5379. DOI:10.1021/acs.analchem.5b00680 |
| [37] |
Q.L. Guo, S. Lin, Y.N. Wang, et al., Chin. Chem. Lett. 27 (2016) 1577-1581. DOI:10.1016/j.cclet.2016.06.040 |
| [38] |
Z. Liu, W. Wang, N. Feng, et al., Acta Pharm. Sin. B 6 (2016) 189-197. DOI:10.1016/j.apsb.2016.03.009 |
| [39] |
D.W. Li, Q.L. Guo, X.H. Meng, et al., Chin. Chem. Lett. 27 (2016) 1745-1750. DOI:10.1016/j.cclet.2016.08.006 |
| [40] |
M. Chen, L. Gan, S. Lin, et al., J. Nat. Prod. 75 (2012) 1167-1176. DOI:10.1021/np3002833 |
| [41] |
M. Chen, S. Lin, L. Li, et al., Org. Lett. 14 (2012) 5668-5671. DOI:10.1021/ol302660t |
| [42] |
X. Wang, M. Chen, F. Wang, et al., Chin. J. Chin. Mater. Med. 38 (2013) 1172-1182. |
| [43] |
Y.F. Liu, M.H. Chen, X.L. Wang, et al., Chin. Chem. Lett. 26 (2015) 931-936. DOI:10.1016/j.cclet.2015.05.052 |
| [44] |
Y.F. Liu, M.H. Chen, Q.L. Guo, et al., J. Asian Nat. Prod. Res. 17 (2015) 689-704. DOI:10.1080/10286020.2015.1055729 |
| [45] |
Y.F. Liu, M.H. Chen, S. Lin, et al., J. Asian Nat. Prod. Res. 18 (2016) 1-12. DOI:10.1080/10286020.2015.1117452 |
| [46] |
Y. Liu, X. Wang, M. Chen, et al., Acta Pharm. Sin. B 6 (2016) 141-147. DOI:10.1016/j.apsb.2016.01.003 |
| [47] |
M.H. Chen, S. Lin, Y.N. Wang, et al., Chin. Chem. Lett. 27 (2016) 643-648. DOI:10.1016/j.cclet.2016.01.042 |
| [48] |
Y. Liu, M. Chen, Q. Guo, et al., Acta Pharm. Sin. B 7 (2017) 179-184. DOI:10.1016/j.apsb.2016.09.004 |
| [49] |
L. Meng, Q. Guo, Y. Liu, et al., Acta Pharm. Sin. B 7 (2017) 334-341. DOI:10.1016/j.apsb.2017.04.003 |
| [50] |
L.J. Meng, Q.L. Guo, C.B. Xu, et al., J. Asian Nat. Prod. Res. 19 (2017) 529-540. DOI:10.1080/10286020.2017.1320547 |
| [51] |
L.J. Meng, Q.L. Guo, C.G. Zhu, C.B. Xu, J.G. Shi, Chin. Chem. Lett. 29 (2018) 119-122. DOI:10.1016/j.cclet.2017.05.019 |
| [52] |
Molecular Operating Environment (MOE 2009. 10), Chemical Computing Group Inc., Montreal, 2009.
|
| [53] |
X.C. Li, D. Ferreira, Y.Q. Ding, Curr. Org. Chem. 14 (2010) 1678-1697. DOI:10.2174/138527210792927717 |
| [54] |
G.P. Moss, Pure Appl. Chem. 72 (2000) 1493-1523. DOI:10.1351/pac200072081493 |
 2018, Vol. 29
2018, Vol. 29