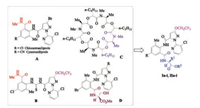
As one class of Ca2+ release channel, ryanodine receptors (RyRs) closely relate to activities inside the insect body [1]. Owing to the unique mode of action towards RyRs, flubendiamide [2, 3], chlorantraniliprole [3, 4] and cyantraniliprole [5] have been developed as three novel insecticides successively since 1999. They exert their pesticidal efficacy through the persistent release of intracellular Ca2+ ion, which ultimately leads to the death of insects. Among those three RyRs insecticides, chlorantraniliprole and cyantraniliprole (Fig. 1, A) both possess an anthranilic diamide structure containing a bromopyrazole ring linked with a chloropyridyl moiety. Due to their excellent insecticidal and ecofriendly properties, the studies on various modifications of such structures have received considerable attention in the last decade [6-12].
|
Download:
|
| Fig. 1. Design strategy of the title compounds. | |
It was reported that more than half of commercial pesticides are fluorinated among which 50% percentage are insecticides and acaricides, during 2010-2016 [13]. This situation may be because that the introduction of fluorine atom could greatly influence the biological activities of the parent molecule owing to the good hydrophobility, permeability, stability and combining ability of the fluorinated compound [14]. For example, novel insecticides and acaricides sulfoxaflor [15], flupyradifurone [16] and pyflubumide [17] all have fluorine-containing substituents just as flubendiamide (contains a heptafluoroisopropyl group). Moreover, some trifluoroethoxyl-containing anthranilic diamide derivatives, e.g., compound B in Fig. 1, also exhibited perfect insecticidal activities against M. separate, P. xylostella, etc. [10, 18-19].
In addition, FKI-1033 (Fig. 1, C) was reported to have activation effect on RyRs and exhibited insecticidal activity [20]. Based on the structural features of chiral α-amino acid ester moieties in FKI-1033, and chiral amide moiety in compound D (Fig. 1) that were derived from our recent researches, the D-amino acid moieties were considered to be favorable for retaining insecticidal activity [7, 21]. In view of all those information mentioned above, a series of new anthranilic diamides containing trifluoroethoxyl and chiral amino acid moieties were synthesized as showed in Fig. 1. The insecticidal activities of the new compounds against oriental armyworm and diamondback moth were evaluated. The preliminary structure-activity relationship (SAR) was also discussed.
The melting points were determined on an X-4 binocular microscope melting point apparatus (Beijing Tech Instrument Co., Beijing, China) and were uncorrected. 1H NMR and 13C NMR spectra were recorded at 400 MHz using a Bruker AV 400 spectrometer (Bruker Co., Switzerland) in CDCl3 or DMSO-d6 solution with tetramethylsilane (TMS) as the internal standard. Elemental analyses were performed on a Vario EL elemental analyzer (Elementar Co., Germany). Optical rotations were measured with a PerkinElmer 341 polarimeter (PE-PerkinElmer Co., Ltd, U.S.A.). Column chromatography purification was carried out using silica gel (200-300 mesh). All chemical reagents and solvents were of analytical grade.
3-(2, 2, 2-Trifluoroethoxy)-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid 6 was synthesized referring to literature (Scheme S1 in Supporting information) [17]. The chiral amino acid esters 9a-f were obtained by the reaction of corresponding D-amino acid with various alcohols (Scheme S2 in Supporting information) [22].
2-(3-(2, 2, 2-Trifluoroethoxy)-1-(3-chloropyridin-2-yl)-1H-pyrazol-5-yl)-6-chloro-8-methyl-4H-benzo[d][1, 3]oxazin-4-one 11a was obtained by reported procedure [19]. 3-(2, 2, 2-Trifluoroethoxy)-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid 6 (10 mmol) and 2-amino-5-chloro-3-methylbenzoic acid 10a (10 mmol) were added into 25 mL of acetonitrile, the mixture was dissolved after the addition of 4 mL of pyridine. And then, 2 mL of methanesulfonyl chloride was added slowly in ice-bath and stirred overnight. After completion of the reaction, the mixture was filtered and the residual solid was the target compound 11a. The compounds 11b and 11c were prepared according to 11a via an "one-pot" method.
(R)-Methyl 2-(2-(3-trifluoroethoxyl-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carbox-amido)-5-chloro-3-methylbenzamido) propanoate Ⅰa was prepared referring to the following procedure (Scheme S3 in Supporting information) [23]. 11a (1.0 mmol) and (R)-methyl 2-aminopropanoate hydrochloride 9a (1.2 mmol) was mixed with 10 mL of tetrahydrofuran, followed by the addition of triethylamine (2.5 mmol). The reaction mixture was stirred overnight. After completion of the reaction, the mixture was filtered and the filtrate was evaporated in vacuum and purified on silica gel eluting with petroleum/ethyl acetate (1:1, v/v) to give Ⅰa. Compounds Ⅰb-i were synthesized using similar procedure to that of Ⅰa.
Using intermediates 11(a-c) and 9(d-f) as materials with similar procedure for Ⅰa, the title compounds (5-substitued-2-(1-(3-chloropyridin-2-yl)-3-(2, 2, 2-trifluoroethoxy)-1H-pyrazole-5-carboxamido)-3-methylbenzoyl)serinate Ⅱ(a-i) were obtained (Scheme S3).
All of the title compounds were identified by melting points, 1H NMR, 13C NMR, elemental analysis and specific optical rotation analysis (Supporting information). In the 1H NMR spectra of compounds Ⅰa-i and Ⅱa-i, the proton signals of two amide groups "—NHCO—" appeared at δ 10.42-9.75 as a singlet peak and δ 8.52-8.40 as a dd peak, respectively. The protons in trifluoroethoxyl group (—OCH2CF3) were observed at δ 4.72-4.62 as a mixed peak with the tertiary hydrogen (—CH) in chiral carbon. From the 13C NMR spectra of the title compounds, it was found that both of the carbons in —OCH2CF3 group were split by the fluorine atoms as quartet, with the coupling constant (J) showed up at 35 Hz for CH2 and 278 Hz for CF3, respectively. From the specific optical rotation analysis, all of the title compounds were dextrorotatory except Ⅰc, Ⅰf, Ⅱe, Ⅱf and Ⅱh.
All of the biological assays were experimented on representative test organisms reared in laboratory. The test insects were cultured according to literatures [24, 25]. The bioassay was replicated at 25 ± 1 ℃ according to statistical requirements. Assessments were based on a dead/alive scale, and mortality rates were rectified by Abbott's formula [26]. Evaluation was based on a percentage scale of 0-100, where 0 equals no activity and 100 equals total kill. Error of the experiments was kept within 5%. For comparative purposes, chlorantraniliprole was tested as a control under the same conditions.
The insecticidal activities of compounds Ⅰa-i, Ⅱa-i and chlorantraniliprole against oriental armyworm (M. separata) were evaluated using reported procedure [27]. For the foliar armyworm tests, individual corn leaves were placed on moistened pieces of filter paper in Petri dishes. The leaves were then sprayed with the test solution and allowed to dry. The dishes were infested with 10 third-instar oriental armyworm larvae. Percentage mortalities were evaluated 2 days after treatment. Each treatment was performed three times.
The insecticidal activities of all the title compounds against oriental armyworm (M. separata) are listed in Table S1 (Supporting information). It was found that all of the newly synthesized compounds Ⅰa-i and Ⅱa-i exhibited remarkable activities against oriental armyworm, e.g., Ⅰa-f, Ⅰh, Ⅱa, Ⅱd and Ⅱh possessed larvicidal activities of 100% at the test concentration of 10 mg/L. In particular, the larvicidal activities of Ⅰa, Ⅰb, Ⅰe, Ⅱd and Ⅱh still displayed mortality rates of 100% at lower concentration, such as 2.5 mg/L. At 1 mg/L, Ⅰa and Ⅱd exhibited 60% and 70% insecticidal activity, respectively. Pleasingly, Ⅰb, Ⅰe and Ⅱh could lead to the total death (100%) of the test oriental armyworm larvae under such conditions. Moreover, Ⅰb, Ⅰe and Ⅱh remained high larvicidal activities even at 0.5 mg/L concentration, with lethality rate of 60%, 20% and 60%, respectively. On the whole, for the methyl and ethyl esters of D-alanine acid and isopropyl ester of D-serine acid of the title compounds, when the R group was Br, their insecticidal activities towards oriental armyworm could be well retained. When R1 was fixed as Cl atom, methyl ester of D-alanine acid and ethyl ester of D-serine acid were superior to others; when R1 was fixed as Br, methyl esters of D-alanine acid were more effective than D-serine acid. Surprisingly, the sequence of insecticidal activity was reversed in the case of isopropyl esters derivatives. The LC50 and LC95 values of compounds Ⅰb, Ⅰe and chlorantraniliprole against oriental armyworm (M. separate) are listed in Table 1. From the results, LC50 of compounds Ⅰb and Ⅰe were 4.68 × 10-4 mol/L and 9.62 ×10-4 mol/L, which exhibited lower efficacy against M. separate than the control chlorantraniliprole (1.37 ×10-4 mol/L). Similarly, the LC95 of Ⅰb, Ⅰe and chlorantraniliprole were 1.71 ×10-3 mol/L, 2.68 × 10-3 mol/L and 0.67 ×10-3 mol/L that there is a gap in the biological activities against oriental armyworm (M. separate) between target compounds Ⅰb, Ⅰe and chlorantraniliprole. In spite of being less active than the control chlorantraniliprole at this preliminary research, the promising compounds Ⅰb, Ⅰe and Ⅱh may be made further structural optimization for the discovery of novel insecticidal agrochemical based on these useful clues.
|
|
Table 1 LC50 and LC95 values of compounds Ⅰb and Ⅰe against oriental armyworm (M. separata). |
The insecticidal activities of Ⅰa, Ⅰb, Ⅰe, Ⅱd and Ⅱh whose mortality rates were 100% against oriental armyworm at 2.5 mg/L were further tested against diamondback moth (P. xylostella) by the leafdip method [28, 29]. A solution of each test sample in DMSO at a concentration of 200 mg/L was prepared and then diluted to the required concentration with water (distilled). Leaf disks (6 cm × 2 cm) were cut from fresh cabbage leaves and then were sprayed with the test solution for 3 s and allowed to dry. The treated leaf disks were placed individually into glass tubes. Each disk was infested with 30 s-instar diamondback moth larvae. Percentage mortalities were evaluated 2 days after treatment. Each treatment was performed in triplicate.
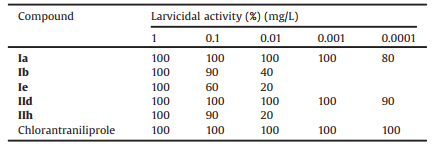
The insecticidal activities of the title compounds Ⅰa, Ⅰb, Ⅰe, Ⅱd and Ⅱh against diamondback moth (P. xylostella) are listed in Table 2. We can see that all of the tested compounds exhibited mortality rate of 100% against diamondback moth at 2.5 mg/L concentration. Especially, at the lower concentration of 0.0001 mg/L Ⅰa and Ⅱd still held larvicidal activities of 80% and 90%, respectively, which result nears to that of chlorantraniliprole. It was found that when the R group was fixed as Cl, the compounds showed favorable activity for D-alanine acid methyl ester and Dserine acid ethyl ester towards diamondback moth. Anyhow, this type of compounds are well worth for further study due to their excellent insecticidal activities.
|
|
Table 2 Insecticidal activities of the title compounds Ⅰa, Ⅰb, Ⅰe, Ⅱd and Ⅱh against diamondback moth (P. xylostella). |
In summary, 18 novel anthranilic diamide compounds containing trifluoroethoxyl group and chiral amino acid moieties were designed, synthesized and evaluated for insecticidal activities against oriental armyworm (M. separata) and diamondback moth (P. xylostella). Most of the title compounds were found to have considerable larvicidal activities towards oriental armyworm, especially Ⅰb and Ⅱh held mortality rate of 60% at 0.5 mg/L. The derivatives with methyl or ethyl ester of D-alanine acid and isopropyl ester of D-serine acid with Br atom of R group could achieve high insecticidal activity against oriental armyworm. For the bioassay against diamondback moth, Ⅰa and Ⅱd exhibited 80%-90% super high larvicidal activity even at the test concentration of 0.0001 mg/L. The test results indicated that D-alanine acid methyl ester and D-serine acid ethyl ester with Cl of R group could retain the similar insecticidal activities as that of chlorantraniliprole against diamondback moth. From the whole results of biological assay, the insecticidal activities of these title compounds may be co-regulated by both chiral amino acid ester and halogen or cyano groups (X group), comparing with previous studies [7]. In addition, the introduction of fluorine-containing group could efficiently retain the high insecticidal activity of such chiral anthranilic diamide compounds. This preliminary research will undoubtedly provide useful information for developing new RyRs activators and insecticidal agrochemicals.
AcknowledgementsThis work was supported by the National Natural Science Foundation of China (Nos. 21772103, 21602118), the National Key Research and Development Program of China (No. 2017YFD0200505) and Tianjin Natural Science Foundation (No. 17JCYBJC19900).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.10.022.
| [1] |
F. Michael, A.C. Julio, Physiol. Rev. 82 (2002) 893-992. DOI:10.1152/physrev.00013.2002 |
| [2] |
T. Masaki, N. Yasokawa, M. Tohnishi, et al., Mol. Pharmacol. 69 (2006) 1733-1739. DOI:10.1124/mol.105.020339 |
| [3] |
P.L. George, C. Daniel, D.B. James, Bioorg. Med. Chem. 17 (2009) 4127-4133. DOI:10.1016/j.bmc.2009.01.018 |
| [4] |
P.L. George, M.S. Thomas, P.S. Thomas, et al., Bioorg. Med. Chem. Lett. 17 (2007) 6274-6279. DOI:10.1016/j.bmcl.2007.09.012 |
| [5] |
K. A. Hughes, G. P. Lahm, T. P. Selby, T. M. Stevenson, Patent, WO2004067528A1, 2004. http://europepmc.org/patents/PAT/HK1111143
|
| [6] |
S.H. Kang, B.A. Song, J. Wu, et al., Eur. J. Med. Chem. 67 (2013) 14-18. DOI:10.1016/j.ejmech.2013.06.023 |
| [7] |
M. Mao, Y. Li, Q. Liu, et al., Bioorg. Med. Chem. Lett. 23 (2013) 42-46. DOI:10.1016/j.bmcl.2012.11.045 |
| [8] |
Y.Y. Zhou, B.L. Wang, F.J. Di, et al., Bioorg. Med. Chem. Lett. 24 (2014) 2295-2299. DOI:10.1016/j.bmcl.2014.03.077 |
| [9] |
X.L. Zhang, Y.X. Li, J.L. Ma, et al., Bioorg. Med. Chem. 22 (2014) 186-193. DOI:10.1016/j.bmc.2013.11.038 |
| [10] |
B.L. Wang, H.W. Zhu, Y. Ma, et al., J. Agric. Food Chem. 61 (2013) 5483-5493. DOI:10.1021/jf4012467 |
| [11] |
C.C. Wu, B.L. Wang, J.B. Liu, et al., Chin. Chem. Lett. 28 (2017) 1248-1251. DOI:10.1016/j.cclet.2017.01.019 |
| [12] |
S. Zhou, S. Zhou, Y.T. Xie, et al., Chin. Chem. Lett. 28 (2017) 1499-1504. DOI:10.1016/j.cclet.2017.02.021 |
| [13] |
P. Jeschke, Pest Manag. Sci. 73 (2017) 1053-1066. DOI:10.1002/ps.2017.73.issue-6 |
| [14] |
T. George, Fluorine containing agrochemicals: an overview of recent developments, in: A. Tressaud (Ed.), Fluorine and the Environment, Elsevier, New York, 2006, pp. 121-127. https://www.mendeley.com/research-papers/fluorinecontaining-agrochemicals-overview-recent-developments/
|
| [15] |
J.M. Babcock, C.B. Gerwick, J.X. Huang, et al., Pest Manag. Sci. 67 (2011) 328-334. DOI:10.1002/ps.v67.3 |
| [16] |
R. Nauen, P. Jeschke, R. Velten, et al., Pest Manag. Sci. 71 (2015) 850-862. DOI:10.1002/ps.3932 |
| [17] |
T. Furuya, A. Suwa, M. Nakano, et al., J. Pestic. Sci. 40 (2015) 38-43. DOI:10.1584/jpestics.D14-087 |
| [18] |
Y. Zhao, Y.Q. Li, L.X. Xiong, H.X. Wang, Z.M. Li, Chin. J. Chem. 30 (2012) 1748-1758. DOI:10.1002/cjoc.v30.8 |
| [19] |
G.P. Lahm, T.M. Stevenson, T.P. Selby, et al., Bioorg. Med. Chem. Lett. 17 (2007) 6274-6279. DOI:10.1016/j.bmcl.2007.09.012 |
| [20] |
S. Omura, K. Shiomi, R. Masuma, Patent, WO2004044214A1, 2004. http://www.freepatentsonline.com/WO2004044214.html
|
| [21] |
S. Zhou, S. Zhou, X.W. Hua, et al., Chin. J. Chem. 34 (2016) 1218-1224. DOI:10.1002/cjoc.v34.12 |
| [22] |
C. Carboni, P.J. Quaedflieg, Q.B. Broxterman, P. Linda, L. Gardossi, Tetrahedron Lett. 45 (2004) 9649-9652. DOI:10.1016/j.tetlet.2004.10.153 |
| [23] |
X.W. Hua, W.T. Mao, Z.J. Fan, et al., Aust. J. Chem. 67 (2014) 1491-1503. DOI:10.1071/CH13701 |
| [24] |
Z.Y. Lu, H.F. Ran, W.X. Liu, Z.G. Qu, J.C. Li, J. Hebei Agric. Sci. 18 (2014) 57-61. |
| [25] |
Z.J. Guo, S. Kang, Q.J. Wu, Y.J. Zhang, Chin. J. Appl. Entomol. 52 (2015) 492-497. |
| [26] |
W.S. Abbott, J. Econ. Entomol. 18 (1925) 265-267. |
| [27] |
L. Chen, Z. Huang, Q. Wang, et al., J. Agric. Food. Chem. 55 (2007) 2659-2663. DOI:10.1021/jf063564g |
| [28] |
H. Ma, K.Y. Wang, X.M. Xia, Y. Zhang, Q.L. Guo, Mod. Agrochem. 5 (2006) 44-46. |
| [29] |
J. R. Busivine, Recommended Methods for Measurement of Pest Resistance to Pesticides, Food and Agriculture Organization of the United Nations, Italy, 1980 pp. 3-13 and 119-122. http://www.researchgate.net/publication/37887619_Recommended_methods_for_measurement_of_resistance_to_pesticides
|
 2018, Vol. 29
2018, Vol. 29