b University of Chinese Academy of Sciences, Beijing 100049, China
Catalytic asymmetric aldol reactions are a crucial carboncarbon formation reaction [1-7]. Since (S)-proline catalyzed asymmetric intermolecular aldol reaction was reported by List, Lerner, and Barbas in 2000 [8, 9], its ready availability and low-cost make it a general method to prepare chiral aldol adducts even to date. High yield or selectivity of List-Lerner-Barbas (LLB) asymmetric aldol reactions was constantly pursued by modifying the structures of catalysts or exploring better experimental conditions [10, 11]. To efficiently improve the yield and selectivity of the catalytic asymmetric LLB reactions, an alternative strategy with suitable additives in the reaction system was usually adopted. So far, some additives including water [12-15], (R/S)-bi-2-naphthol (BINOL) [16], Schreiner's thiourea [17] and other diarylthioureas [18, 19] have been reported. However, developing new additive is still attractive, especially for the substrates, which could not be efficiently performed under the typical proline-catalyzed asymmetric LLB aldol conditions.
Chiral diols such as (R/S)-BINOL were often used as catalysts or additives in many asymmetric catalytic reactions [20, 21]. However, no helicenediols and their analogues as catalysts or additives used in LLB-A reactions have been reported so far [22-24]. Recently, we synthesized a series of THB-[5]HDIOL derivatives, and found they could be utilized as chiral fluorescence sensors for the enantioselective recognition of tryptophan methyl esters [25]. Herein, we report an efficient proline-catalyzed asymmetric LLB aldol reactions of bulky aromatic aldehyde substrates with THB-[5] HDIOL derivatives as additives.
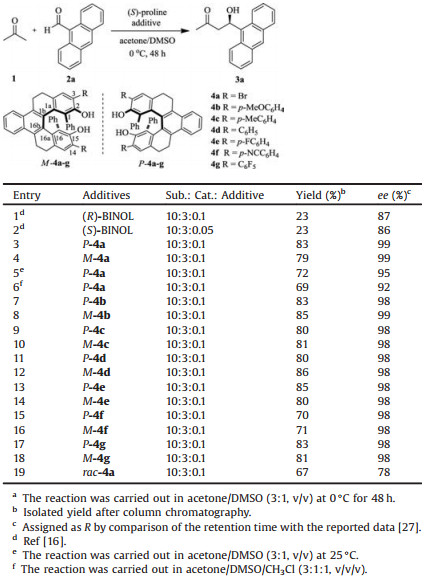
Based on the crystal structure of enantiomer P-4a [25], the distance between the two oxygen atoms of hydroxyl groups is 5.65 Å vs. 3.33 Å in BINOL [26]. Therefore, a more commodious chiral environment of P-4a might be expected, which encouraged us to test P-4a as an additive for the proline-catalyzed LLB-AA reactions of 9-anthraldehyde and acetone. It was found that when ([R/S)-BINOL was used as the additive for the LLB-A reaction, 23% yield and 86% ee of (4R)-hydroxy-4-(9'-anthranyl)- butan-2-one 3a was only obtained (Table 1, entry 1) [16]. But with helical diol P-4a as an additive, the reaction between 1 and 2a could proceed smoothly under similar conditions to give the product in 83% yield and 99% ee (Table 1, entry 3). Moreover, an increase in temperature (Table 1, entry 5) or with the mixture of DMSO and CHCl3 (1:1) as cosolvent (Table 1, entry 6) could result in decrease of the enantioselectivity. We also found that the electronic effect of the substituents at 3, 14-positions of the helical scaffold exerted little influence on the reaction outcomes. Consequently, similar yields and enantioselectivities were found for the diols with either electron donating substituent such as P/M-4b (R = p-MeOC6H4) and P/M-4c (R = p-MeC6H4) or electron withdrawing substituents [P/M-4e-g, R = p-FC6H4, p-NCC6H4 or C6F5] (Table 1, entries 7–18). Obvious low yield and enantioselectivity was obtained with rac-4a which indicated chiral THB-[5] HDIOL derivatives as additives was crucial. (Table 1, entry 19). It was also found that the reaction would not be carried out without the (S)-proline. Taking previous reports into account [16], it was concluded that the chiral additives THB-[5]HDIOL could enhance the chiral inductive ability of (S)-proline and the stability of the system by formation of a chiral supramolecular system through hydrogen-bonding interactions. The chirality of the product was controlled by the (S)-proline, while chiral THB-[5]HDIOL was used as chiral additives to increase the enantioselectivity in this reaction.
|
|
Table 1 (S)-Proline catalyzed LLB-AA reactions with the diols as additivea. |
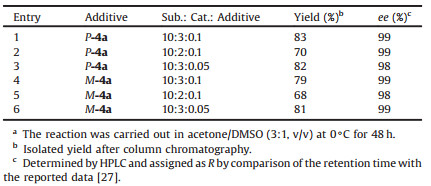
To improve the activity and stereoselectivity of the reaction, the catalyst and the additive loading were then screened. The results were summarized in Table 2. It was found that with P-4a as additive, 20 mol% loading amount of proline led to the unchanged enantioselectivity but significantly lower yield (Table 2, entry 2). With 0.5 mol% P-4a as additive, the yield and enantioselectivity decreased in a small range (Table 2, entry 3). If M-4a was used as additive, reducing the loading amount of proline led to lower yield and enantioselectivity while 0.5 mol% M-4a could give better enantioselectivity and yield (Table 2, entries 5 and 6). Overall, taking the yield and enantioselectivity into consideration, the reaction of 9-anthraldehyde 2a with acetone was carried out at 0℃ by using 30 mol% proline as catalyst and 1.0 mol% P-4a as additive to produce 3a in 83% yield with 99% ee. Alternatively, when 0.5 mol% M-4a was chosen as additive, 81% yield and 99% ee of the product could be obtained.
|
|
Table 2 Screening of (S)-proline and additive loadings on the LLB-AA reactiona. |
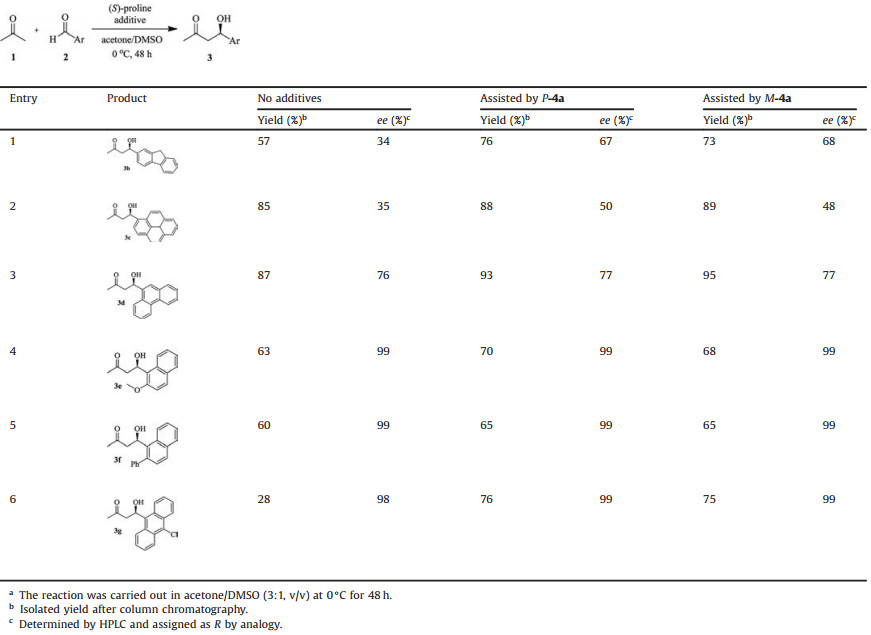
Under the optimization conditions for LLB-AA reaction of the 9-anthraldehyde, we further shifted our attention onto the other bulky aromatic aldehydes. As shown in Table 3, it was found that compared with no additive, the yields and enantioselectivities for the LLB-AA reactions of 2-fluorenecarboxaldehyde (3b) and 1-pyrenecarboxaldehyde (3c) in the presence of additives P-4a and M-4a were significantly high (Table 3, entries 1 and 2). In the case of 9-phenanthrenecarboxaldehyde (3d), the additives led to higher yield but showed no significant effect on the stereoselectivity (Table 3, entry 3). It was also found that the additives could accelerate the LLB-AA reactions for substituted naphthaldehyde derivatives 3e-f and anthraldehyde derivative 3g, and the yields could be obviously improved (Table 3, entries 4–6).
|
|
Table 3 LLB-AA reactions with different bulky aldehydes in the presence of (S)-proline and additives a. |
Density functional theory (DFT) calculations at the M06-2X/ 6-31+G(d, p) (LANL2DZ for Br) level of theory were also carried out to gain further insight into the origin of the increased enantioselectivity. The optimized TS geometries conveyed some vital details that help rationalize this energy difference. Two ring transition states TS(R) and TS(S) including a simultaneous C—C bond formation procedure alongside with a hydrogen-shift, leading to the R- and S-configured products, respectively, were located in Fig. 1. It was found TS(R) was favored over TS(S) by 3.2 kcal/mol in terms of the activation Gibbs free energy in the gas phase, indicating that the R-configured product was kinetically favored, which is consistent with our experimental observations. It was found that more favorable transition states TS (R) was stabilized by seven hydrogen bonding and a CH-Br interaction between the diol and substrates (for detailed analysis, see Supporting information, AIM Part). Most importantly, the anthracenyl group of 9-anthraldehyde in TS(R) was almost perpendicular to the phenyl group of the additive, indicating the presence of an extra T-shaped π-π stacking interaction which played pivotal role. Such π-π stacking interaction was absent in the competing transition state TS(S) and consequently, TS(S) is not favored in this addition reaction. Furthermore, the edge-to-face π-π stacking may contribute mainly for the 3.2 kcal/mol difference in the gas phase.

|
Download:
|
| Fig. 1. Optimized geometries of the (a) TS(R) and (b) TS(S) transition states at the M06-2X/6-31+G(d, p) (LANL2DZ for Br) level of theory. The relative free energies in the gas phase (kcal/mol) are provided in parentheses. | |
In summary, we have demonstrated efficient proline-catalyzed LLB-AA reactions of bulky aldehyde substrates with THB-[5]HDIOL derivatives as additives, and obtained the aldol product of 9- anthraldehyde and acetone in up to 83% yield and 99% ee, which were obviously higher than the reported results with (R)- or (S)- BINOL as the additives. For other tested bulky aromatic aldehyde substrates, the similar improved yields and enantioselectivities of the LLB-AA reactions were also obtained. The results presented herein can expand an opportunity for the helical diols to be used in asymmetric catalysis, in particular for bulky substrates involved reactions.
AcknowledgmentsWe thank Dr. Wei Meng from Institute of Chemistry, CAS for his helps in the calculations. We also thank the National Natural Science Foundation of China (No. 21572233), and the Strategic Priority Research Program of Chinese Academy of Sciences (No. XDB12010400) for financial supports.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.06.007.
| [1] |
S.B.J. Kan, K.K.H. Ng, I. Paterson, Angew. Chem. Int. Ed. 52 (2013) 9097-9108. DOI:10.1002/anie.201303914 |
| [2] |
S. Adachi, T. Harada, Eur. J. Org. Chem. (2009) 3661-3671. |
| [3] |
W. Qi, X. Xie, T. Zhong, X. Zhang, Chin. Chem. Lett. 29 (2018) 194-196. DOI:10.1016/j.cclet.2017.04.019 |
| [4] |
R. Tao, X.J. Yin, K.H. Wang, et al., Chin. Chem. Lett. 26 (2015) 1046-1049. DOI:10.1016/j.cclet.2015.04.015 |
| [5] |
L. Zhang, W.B. Ding, Y.P. Yu, H.B. Zou, Chin. Chem. Lett. 20 (2009) 1065-1067. DOI:10.1016/j.cclet.2009.04.034 |
| [6] |
X.D. Zhang, N. Gao, Z. Guan, Y.H. He, Chin. Chem. Lett. 27 (2016) 964-968. DOI:10.1016/j.cclet.2016.02.013 |
| [7] |
L.J. Jiang, Z.G. Zhang, Chin. J. Org. Chem. 26 (2006) 618-626. |
| [8] |
B. List, R.A. Lerner, C.F. Barbas, J. Am. Chem. Soc. 122 (2000) 2395-2396. DOI:10.1021/ja994280y |
| [9] |
W. Notz, B. List, J. Am. Chem. Soc. 122 (2000) 7386-7387. DOI:10.1021/ja001460v |
| [10] |
Z. Tang, Z.H. Yang, X.H. Chen, et al., J. Am. Chem. Soc. 127 (2005) 9285-9289. DOI:10.1021/ja0510156 |
| [11] |
Z. Tang, F. Jiang, L.T. Yu, et al., J. Am. Chem. Soc. 125 (2003) 5262-5263. DOI:10.1021/ja034528q |
| [12] |
M. Amedjkouh, Tetrahedron:Asymmetry 16 (2005) 1411-1414. DOI:10.1016/j.tetasy.2005.02.031 |
| [13] |
D.E. Ward, V. Jheengut, Tetrahedron Lett. 45 (2004) 8347-8350. DOI:10.1016/j.tetlet.2004.09.061 |
| [14] |
P.M. Pihko, K.M. Laurikainen, A. Usano, A.I. Nyberg, J.A. Kaavi, Tetrahedron 62 (2006) 317-328. DOI:10.1016/j.tet.2005.09.070 |
| [15] |
P. Pihko, A. Nyberg, A. Usano, Synlett 2004 (2004) 1891-1896. DOI:10.1055/s-2004-831296 |
| [16] |
Y. Zhou, Z. Shan, J. Org. Chem. 71 (2006) 9510-9512. DOI:10.1021/jo060802y |
| [17] |
O. Reis, S. Eymur, B. Reis, A.S. Demir, Chem. Commun. (2009) 1088-1090. |
| [18] |
El-Hamdouni N., X. Companyó, R. Rios, A. Moyano, Chem.-Eur. J. 16 (2010) 1142-1148. DOI:10.1002/chem.v16:4 |
| [19] |
X. Companyó, G. Valero, L. Crovetto, A. Moyano, R. Rios, Chem.-Eur. J. 15 (2009) 6564-6568. DOI:10.1002/chem.v15:27 |
| [20] |
Y. Chen, S. Yekta, A.K. Yudin, Chem. Rev. 103 (2003) 3155-3212. DOI:10.1021/cr020025b |
| [21] |
J.D. Chen, L. Fang, C.F. Chen, Mini-Rev. Org. Chem. 12 (2015) 310-327. DOI:10.2174/1570193X12666150930224015 |
| [22] |
Y. Shen, C.F. Chen, Chem. Rev. 112 (2012) 1463-1535. DOI:10.1021/cr200087r |
| [23] |
W.B. Lin, M. Li, L. Fang, C.F. Chen, Chin. Chem. Lett. 29 (2018) 40-46. DOI:10.1016/j.cclet.2017.08.039 |
| [24] |
L. Fang, W.B. Lin, M. Li, C.F. Chen, Chin. J. Org. Chem. 38 (2018) 541-554. DOI:10.6023/cjoc201710028 |
| [25] |
L. Fang, M. Li, W.B. Lin, Y. Shen, C.F. Chen, J. Org. Chem. 82 (2017) 7402-7409. DOI:10.1021/acs.joc.7b01087 |
| [26] |
K. Mori, Y. Masuda, S. Kashino, Acta. Cryst. C 49 (1993) 1224-1227. DOI:10.1107/S0108270193000083 |
| [27] |
A. Martinez, van Gemmeren M., B. List, Synlett 25 (2014) 961-964. DOI:10.1055/s-00000083 |
 2018, Vol. 29
2018, Vol. 29