Supramolecular hydrogels formed by low-molecular-weight gelators (LMWGs), especially biomolecules possessing nice biocompatibility, have aroused a great deal of interest over the past few decades [1-7]. The self-assembly of LMWGs is driven by non-covalent interactions, such as hydrogen bonding, π–π interactions, metal-ligand coordination, van der Waals forces. With the deep understanding of the molecular interactions, the design of supramolecular hydrogels with desired functionality becomes possible. Based on the properties of gelators, various nanostructures could be obtained, nanofibers commonly [8-10], but also nanobelts, nanotwists [11], nanotubes [12] and nanospheres [13], and various functions could be developed in sensors [14], catalysis [15], optoelectronics [16], wound healing [17, 18], drug delivery and tissue engineering [19, 20].
Among the various class of biomolecules, derivatives of amino acids, designed with bespoke functionality, are a highly promising area of recent development [21-24], given that amino acids, components that construct a living body, have biocompatibility, biodegradation and non-toxicity. In addition, amino acids are commercially available at a relatively low price, which provide convenience for the mass synthesis of their derivatives. A series of amino acid derivatives, including alanine, histidine, proline, glutamic acid and so on, were prepared to construct various self-assembled structures, in some cases leading to twist or helical nanostructures with supramolecular chirality [25-32]. The development of supramolecular gels would facilitate the preparation of derivatives of amino acids.
Since minor changes in molecular structure can impact the macroscopic characteristics [33-36], such as viscosity and elasticity, which is closely related to the hydrophilic and hydrophobic structures of the gelators used, the present work focuses on the effect of molecular structure on the self-assembly properties. We designed three histidine-derived surfactants with different alkyl chain length, NIPCA, UIPCA, TIPCA (Scheme 1), which were synthesized through Pictet-Spengler reaction (Supporting information). We chose L-histidine as a platform of LMWGs based on several reasons: First, L-histidine, one of amino acids, has nice biocompatibility and biodegradation and can be widely used in vivo applications. Second, L-histidine has four hydrogen bonding sites, such as amido and carboxyl, which contributes to the supramolecular self-assembly. Third, synthetic procedures of L-histidine derivatives by the introduction of various functional groups are simple and established [26-29, 37]. We found that all these three derivatives could self-assemble with citric acid (CA), leading to hydrogels with different characteristics. Moreover, the hydrogels formed by UIPCA with CA could incorporate some dyes with good capacity, and showed high release selectivity which could be used in the separation of oppositely charged dyes.

|
Download:
|
| Scheme 1. The chemical structures of histidine-derived amphiphiles with different chain length: 4-nonyl-4, 5, 6, 7-tetrahydro-1H-imidazo[4, 5-c]pyridine-6-carboxylic acid (NIPCA), 4-undecyl-4, 5, 6, 7-tetrahydro-1H-imidazo[4, 5-c]pyridine-6-carboxylic acid (UIPCA) and 4-tridecyl-4, 5, 6, 7-tetrahydro-1H-imidazo[4, 5-c]pyridine-6- carboxylic acid (TIPCA). | |
The gelation properties of the three histidine-derived surfactants with different chain length were studied. One can see that the single surfactants, NIPCA, UIPCA, and TIPCA, all can form hydrogels with citric acid at appropriate concentrations, as shown in Fig. 1 and Fig. S4 in Supporting information. Unlike the enantioselectivity of tartaric acid to UIPCA [37], critic acid shows no selection to the two enantiomers, (4R, 6S)-UIPCA and (4S, 6R)- UIPCA, mostly due to its achiral structure. However, when the racemates of (4R, 6S)-UIPCA and (4S, 6R)-UIPCA were introduced, no hydrogels but only precipitates were formed (Fig. 1e). The hydrogels formed by enantiomers were proved almost the same gelation properties (Figs. 1b and c and Table 1) except the opposite circular dichroism (Fig. S5 in Supporting information). Thus, we mainly detect the gelation behaviors of derivates of L-histidine, i.e., (4R, 6S)-NIPCA, (4R, 6S)-UIPCA, and (4R, 6S)-TIPCA, with citric acid in the present work.

|
Download:
|
| Fig. 1. Photos of hydrogels and the precipitates formed by 600 mmol/L CA with (a) 150 mmol/L NIPCA, (b) 30 mmol/L (4R, 6S)-UIPCA, (c) 30 mmol/L (4S, 6R)-UIPCA, (d) 30 mmol/L TIPCA, and (e) 30 mmol/L racemate of UIPCA at 25 ℃. | |
|
|
Table 1 Gelation behaviors of different compounds with citric acid. |
Fig. 2 shows the aggregation behaviors of NIPCA, UIPCA and TIPCA with 600 mmol/L CA, in which the effect of the chain length on the gelation ability can obviously be found. It is known that delicate balance between the crystallization and dissolution leads to the gelation process which is closely related to the hydrophilic and hydrophobic structures of the gelators used. One can note that at same CA concentrations, the hydrogels occur at lower concentrations of UIPCA than those of NIPCA, exhibiting the stronger gelation ability, which is ascribed to the longer alkyl chain and higher hydrophobicity of UIPCA. TIPCA with the longest chain shows excellent gelation ability. Compared with other two compounds, it can gelate the largest amount of water molecules with the shortest time and the lowest concentration (Table 1).

|
Download:
|
Fig. 2. Phase transition with the addition of (a) NIPCA, (b) UIPCA and (c) TIPCA to 600 mmol/L CA solutions at 25 ℃. Different phases are marked as: solution     |
|
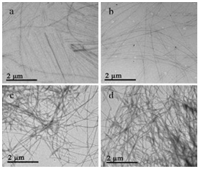
To investigate the morphologies of hydrogels, TEM observations were performed. From the images in Fig. 3, the hydrogels formed by UIPCA with citric acid are characterized by fibrous structures with high aspect ratios, which are physically entangled to form three dimensional (3D) networks. It has also been found that, with the increase in the concentration of UIPCA, the fibers intertwine more densely with the larger diameters, which enhances the mechanical strength of hydrogels. The hydrogels formed by TIPCA (Fig. S6a in Supporting information) and NIPCA (Fig. S6b in Supporting information) show similar fibrous microstructures.
|
Download:
|
| Fig. 3. TEM images of hydrogels formed by 600 mmol/L citric acid with (a) 30, (b) 70, (c) 110 and (d) 140 mmol/L UIPCA. | |
It is essential to investigate the mechanical properties of hydrogels, which are directly related to their applications. The mechanical properties can be reflected by rheological results. Frequency sweep is an important method to detect the tolerance performance of a material to external forces [38, 39]. The frequency sweep (Fig. S7 in Supporting information) measurements indicate that, at a fixed CA concentration, both G' and G" increase with the concentration of UIPCA, due to the more rigid networks caused by denser fiber entanglements. It also shows that G' is always greater than G" within the studied frequency region, 0.01–10 Hz, demonstrating the solid-like behavior of the hydrogels. The G' value reaches more than 105 Pa at 140 mmol/L UIPCA, indicating the quite strong mechanical strength.
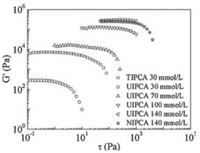
The solid-like networks of hydrogels break down under the critical shear stress, which is also called the "yield stress", being another important parameter to display the mechanical strength of hydrogels. Fig. 4 clearly shows that, at the same concentration, the yield stress decreases in the sequence of TIPCA, UIPCA and NIPCA, indicating that the mechanical strength of hydrogels increases with the increase in chain length. With the increasing concentration of NIPCA and TIPCA, hydrogels exhibit similar rheological properties, suggesting the similar interactional mechanism in spite of the different hydrocarbon chain lengths (Fig. S8 in Supporting information).
|
Download:
|
| Fig. 4. G' as a function of oscillatory stress at cCA = 600 mmol/L with different concentrations of NIPCA, UIPCA and TIPCA. | |
In order to elucidate long range ordered structures of aggregation in gel networks at a molecular level, SXRD measurements of xerogels were performed. As shown in Fig. 5a, the xerogels of NIPCA, UIPCA, and TIPCA show three reflections at 2θ of 3.20°, 2.90°, and 2.34°, respectively, corresponding to the spacing values of 2.73 nm, 3.04 nm, and 3.68 nm, which should be ascribed to the arrangement of molecules with different hydrocarbon chain length. Since these three systems self-assemble in similar way, we choose UIPCA as a model to discuss the interactional mechanism. As the spacing, 3.04 nm, which does not change with the ratio of UIPCA to CA (Fig. S9 in Supporting information), is a little less than twice of the length of a UIPCA molecule, 1.69 nm [37], it is supposed that two UIPCA molecules self-assemble into bilayer structures with a CA molecule in a part overlapping form.

|
Download:
|
| Fig. 5. (a) Small angle XRD spectra of xerogels with different compositions. (b) FT-IR spectra of UIPCA, CA, UIPCA/CA xerogel. The concentration of citric acid of all samples is 600 mmol/L. | |
FT-IR spectroscopy was employed to detect the hydrogen bonding in hydrogels (Fig. 5b). For single UIPCA, only the asymmetric and symmetric stretching vibrations of COO- group at 1625 cm-1 and 1398 cm-1 appear, while the carbonyl stretching band at about 1700 cm-1 cannot be observed, which should be attributed to the dissociation of H+ from carboxyl group, indicating the existence of UIPCA as zwitterionic form. For single CA, the two peaks at 1748 cm-1 and 1705 cm-1 can be assigned to the hydrogen bonded C=O and free C=O on carboxyl group, respectively. While for UIPCA/CA complex, a transition from two peaks to a single peak can be observed, indicating that in the oxerogels, all carbonyl groups on CA molecules are hydrogen bonded C=O.
On the basis of the results from SXRD and FT-IR analysis, the possible formation process of the hydrogel networks of UIPCA was proposed. Since the—NH—group in the tetrahydropyridine ring is protonated by H+ dissociated from the carboxyl group, UIPCA molecule exists as zwitterionic form. After adding UIPCA to CA solution, H+ ions dissociated from CA protonate the—N=group on the imidazole ring of the UIPCA molecules, that suggests the presence of the electrostatic interaction. Besides, the hydrogen bonding between the—COO- groups of UIPCA molecules and—OH groups of CA molecules, and the hydrophobic interaction between the long alkyl chains on UIPCA molecules, together drive the self-assembly of UIPCA with CA molecules. Thus, we can conclude that the derivatives of L-histidine interact with CA via electrostatic interaction, hydrogen bonding and hydrophobic interaction, ultimately leading to fibers that underlie the hydrogel formation.
Interestingly, we found that hydrogels formed by UIPCA with CA could incorporate various dyes with good capacity and exhibit excellent releasing selectivity for charged dyes in aqueous solution (Fig. 6 and Fig. S10 in Supporting information). Anionic dyes, chrome azurol S (CAS) and methyl orange (MO), and cationic dyes, methyl violet (MV) and rhodamine 6G (R6G), were chosen as models for this study. For the anionic dyes, CAS and MO, it was found that nearly no dye was released to water even being kept for 10 days (Fig. 6a and Fig. S10), which has been confirmed by UV–vis spectroscopy (Fig. 6b). In the case of cationic dyes, a wellcontrollable release process was observed from sample photos (Fig. 6a) and UV–vis spectroscopy (Fig. 6c). The selective release of anionic and cationic dyes from hydrogels was further studied. For the mixtures of CAS and MV incorporated in hydrogels, it was found that MV can be separated from dye mixtures gradually, while CAS is retained in the hydrogel completely (Fig. 6d), showing the great selectivity and separating ability of hydrogels for differently charged dyes. Moreover, during the releasing process, the hydrogels could maintain their original shape. Based on these special properties, the present hydrogels might have great potential to achieve the well-controllable stepwise release of multicomponent drugs as drug carriers.

|
Download:
|
| Fig. 6. Release of CAS and MV from hydrogel of 70mmol/L UIPCA/600 mmol/L CA. Photos of hydrogel samples containing CAS (top) and MV (below) with time (a); UV–vis spectra of (b) CAS, (c) MV and (d) mixtures of CAS and MV released from hydrogels. | |
In conclusion, three derivatives of L-histidine with different alkyl chain lengths, NIPCA, UIPCA and TIPCA, were synthesized and their self-assembly with citric acid in aqueous solutions was investigated. The substances all can gelate with citric acid at appropriate concentrations to form hydrogels composed of entangled nanofibers. The gelation ability remarkably decreases in the order of TIPCA, UIPCA and NIPCA, indicating the great effect of alkyl chain on the gelation behavior, for which the longer alkyl chain promotes the gelation ability and enhances the mechanical strength of hydrogels. SXRD and FT-IR reveal that the L-histidine derivatives interact with CA via the synergistic effect of electrostatic interaction, hydrogen bonding and hydrophobic interaction, leading to the formation of entangled fibers and eventually the network structures. Furthermore, hydrogels formed by UIPCA with CA possess excellent ability of the selective release for differently charged dyes. This work provides not only an important clue in the design of supramolecular hydrogels by taking account of the effect of hydrocarbon chain, but also the fascinating potential applications in the selective release or separation of dyes with opposite charges.
AcknowledgmentThis work was funded by the National Natural Science Foundation of China (No. 21573134).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.12.024.
| [1] |
P. Terech, R.G. Weiss, Chem. Rev. 97 (1997) 3133-3159. DOI:10.1021/cr9700282 |
| [2] |
L.A. Estroff, A.D. Hamilton, Chem. Rev. 104 (2004) 1201-1217. DOI:10.1021/cr0302049 |
| [3] |
N. Sangeetha, U. Maitra, Chem. Soc. Rev. 34 (2005) 821-836. DOI:10.1039/b417081b |
| [4] |
P. Dastidar, Chem. Soc. Rev. 37 (2008) 2699-2715. DOI:10.1039/b807346e |
| [5] |
L.E. Buerkle, S.J. Rowan, Chem. Soc. Rev. 41 (2012) 6089-6102. DOI:10.1039/c2cs35106d |
| [6] |
C. Tomasini, N. Castellucci, Chem. Soc. Rev. 42 (2013) 156-172. DOI:10.1039/C2CS35284B |
| [7] |
G. Yu, X. Yan, C. Han, F. Huang, Chem. Soc. Rev. 42 (2013) 6697-6722. DOI:10.1039/c3cs60080g |
| [8] |
P. Xue, R. Lu, G. Chen, et al., Chem.-Eur. J. 13 (2007) 8231-8239. DOI:10.1002/(ISSN)1521-3765 |
| [9] |
T. Tu, L. Bao, W. Assenmacher, et al., Chem.-Eur. J. 15 (2009) 1853-1861. DOI:10.1002/chem.v15:8 |
| [10] |
J.K.H. Hui, Z. Yu, M.J. MacLachlan, Angew. Chem. Int. Ed. 46 (2007) 7980-7983. DOI:10.1002/(ISSN)1521-3773 |
| [11] |
A. Gopal, M. Hifsudheen, S. Furumi, M. Takeuchi, A. Ajayaghosh, Angew. Chem. Int. Ed. 51 (2012) 10505-10509. DOI:10.1002/anie.201205332 |
| [12] |
N. Shi, G. Yin, H. Li, M. Han, Z. Xu, New J. Chem. 32 (2008) 2011-2015. DOI:10.1039/b804455d |
| [13] |
M. Yamanaka, H. Fujii, J. Org. Chem. 74 (2009) 5390-5394. DOI:10.1021/jo900894q |
| [14] |
T. Tu, W. Fang, Z. Sun, Adv. Mater. 25 (2013) 5304-5313. DOI:10.1002/adma.201301914 |
| [15] |
D.D. Díaz, D. Kühbeck, R.J. Koopmans, Chem. Soc. Rev. 40 (2011) 427-448. DOI:10.1039/C005401C |
| [16] |
S.S. Babu, S. Prasanthkumar, A. Ajayaghosh, Angew. Chem. Int. Ed. 51 (2012) 1766-1776. DOI:10.1002/anie.v51.8 |
| [17] |
G.A. Silva, C. Czeisler, K.L. Niece, et al., Science 303 (2004) 1352-1355. DOI:10.1126/science.1093783 |
| [18] |
V. Jayawarna, M. Ali, T.A. Jowitt, et al., Adv. Mater. 18 (2006) 611-614. DOI:10.1002/(ISSN)1521-4095 |
| [19] |
R.N. Shah, N.A. Shah, Del Rosario Lim M.M., et al., Proc. Natl. Acad. Sci. U. S. A. 107 (2010) 3293-3298. DOI:10.1073/pnas.0906501107 |
| [20] |
K.J. Skilling, F. Citossi, T.D. Bradshaw, et al., Soft Matter 10 (2014) 237-256. DOI:10.1039/C3SM52244J |
| [21] |
J. Liu, F. Xu, Z. Sun, et al., Soft Matter 12 (2016) 141-148. DOI:10.1039/C5SM02111A |
| [22] |
D. Das, A. Dasgupta, S. Roy, et al., Chem.-Eur. J. 12 (2006) 5068-5074. DOI:10.1002/(ISSN)1521-3765 |
| [23] |
A. Banerjee, G. Palui, A. Banerjee, Soft Matter 4 (2008) 1430-1437. DOI:10.1039/b802205b |
| [24] |
S. Basak, J. Nanda, A. Banerjee, J. Mater. Chem. 22 (2012) 11658-11664. DOI:10.1039/c2jm30711a |
| [25] |
H. Cao, X. Zhu, M. Liu, Angew. Chem. Int. Ed. 52 (2013) 4122-4126. DOI:10.1002/anie.201300444 |
| [26] |
Y. Liu, T. Wang, M. Liu, Chem.-Eur. J. 18 (2012) 14650-14659. DOI:10.1002/chem.201202637 |
| [27] |
Y. Liu, T. Wang, Y. Huan, et al., Adv. Mater. 25 (2013) 5875-5879. DOI:10.1002/adma.201302345 |
| [28] |
Y. Liu, T. Wang, Z. Li, M. Liu, Chem. Commun. 49 (2013) 4767-4769. DOI:10.1039/c3cc41786g |
| [29] |
L. Wang, Y. Liu, Z. Shen, T. Wang, M. Liu, Chem. Commun. 50 (2014) 15874-15877. DOI:10.1039/C4CC07813F |
| [30] |
L. Qin, L. Zhang, Q. Jin, et al., Angew. Chem. Int. Ed. 52 (2013) 7761-7765. DOI:10.1002/anie.v52.30 |
| [31] |
W. Miao, L. Qin, D. Yang, X. Jin, M. Liu, Chem. -Eur. J. 21 (2015) 1064-1072. DOI:10.1002/chem.201405406 |
| [32] |
W. Miao, D. Yang, M. Liu, J. Chem. Eur. 21 (2015) 7562-7570. DOI:10.1002/chem.201500097 |
| [33] |
A. Pal, J. Dey, Langmuir 27 (2011) 3401-3408. DOI:10.1021/la105027b |
| [34] |
A. Pal, S. Abraham, M.A. Rogers, J. Dey, R.G. Weiss, Langmuir 29 (2013) 6467-6475. DOI:10.1021/la400664q |
| [35] |
A. Pal, J. Dey, Langmuir 29 (2013) 2120-2127. DOI:10.1021/la3042764 |
| [36] |
T. Patra, A. Pal, J. Dey, Langmuir 26 (2010) 7761-7767. DOI:10.1021/la904540x |
| [37] |
F. Zhang, Z. Xu, S. Dong, et al., Soft Matter 10 (2014) 4855-4862. DOI:10.1039/c4sm00479e |
| [38] |
P.D. Sawant, X.Y. Liu, Chem. Mater. 14 (2002) 3793-3798. DOI:10.1021/cm0116875 |
| [39] |
M.G. Page, G.G. Warr, J. Phys. Chem. B 108 (2004) 16983-16989. DOI:10.1021/jp0470602 |
 2018, Vol. 29
2018, Vol. 29 



