Chiral sulfones are important structure motifs in biologically active molecules and versatile building blocks in organic synthesis [1]. Diverse synthetic approaches have been developed for the formation of chiral sulfones including metal-catalyzed hydrogenation [2], asymmetric substitution [3], cycloaddition [4], carbon– hydrogen insertion [5], Lewis acid-mediated Diels–Alder reactions [6], and organocatalytic versions [7]. Recently, our group reported the kinetic resolution of β-sulfonyl ketones through enantioselective β-elimination (Scheme 1a) [8], in which one enantiomer of racemic β-sulfonyl ketones was preferentially eliminated to form α, β-unsaturated enones and deliver enantioenriched β-sulfonyl ketones and achiral enones. In a typical kinetic resolution, unreacted substrates have high enantioselectivities, whereas the scalemic reaction product is generally discarded. Unless reagents with a large s value (≥200) are used to produce enantioenriched products and recover starting materials at 50% conversion [9], it is difficult to realize such levels of enantioselectivities. Besides, in classical kinetic resolution via oxidation and elimination [10], the stereocenters of starting materials are removed to form achiral products. For these reasons, it is difficult to obtain high enantioselectivities of both substrates and products in classical kinetic resolution. To solve the above problems, we hypothesized that adding reactive synthons into the kinetic resolution process might enhance the enantioselectivities of the products or convert achiral products into useful enantioenriched molecules. In the previous report of the asymmetric synthesis of trisubstituted tetrahydrothiophenes (Scheme 1b) [11], commercially available 1, 4-dithiane-2, 5-diol [12] showed the impressive ability in direct construction of trisubstituted tetrahydrothiophenes with enones in the presence of cation-binding catalyst. By introducing 1, 4- dithiane-2, 5-diol to the simple kinetic resolution of β-sulfonyl ketones, two asymmetric reactions including kinetic resolution of β-sulfonyl ketones and cascade sulfa-Michael/Aldol reaction proceeded successively to afford chiral sulfonyl ketones and tetrahydrothiophenes. In this strategy, the stereocontrol of the two asymmetric reactions in one-pot by means of one ion-pairing catalyst remains a great challenge.

|
Download:
|
| Scheme 1. Enantioselective one-pot synthesis of β-sulfonyl ketones and trisubstituted tetrahydrothiophenes. | |
To verify our hypothesis, we initiated the synthesis with racemic 3-(4-fluorophenyl)-1-phenyl-3-(phenylsulfonyl)propan- 1-one 2f as a model substrate in the presence of 1, 4-dithiane- 2, 5-diol and potassium salt as base as well as Song's oligoEG catalyst [13]. The effect of potassium salt was firstly examined in DCM (Table 1, entries 1–5). As expected, the one-pot reaction sequence including kinetic resolution of β-sulfonyl ketones and cascade sulfa-Michael/Aldol reaction proceeded smoothly to afford β-sulfonyl ketones (R)-2f and trisubstituted tetrahydrothiophenes 4f in moderate to high enantioselectivities when the potassium salts were KOCN, phthalimide potassium, KF, and KOAc (Table 1, entries 1–4). Among them, KF showed the best selectivity (s = 13), providing both (R)-2f and 4f in high stereoselectivities (92%, 91%) (Table 1, entry 3). Otherwise, when the bases were KCl, KBr, KI, KSO2Ph, KPF6, KBF4, K2S2O8, or KSCN, the reaction did not take place (Table 1, entry 5). In further investigation of solvents, nonpolar solvents (toluene, DCE and CHCl3) and polar solvents (THF and 1, 4-dioxane) showed the worse selectivity (Table 1, entries 6–10).
|
|
Table 1 Optimization of the reaction conditions.a |
Under the optimal reaction conditions (Table 1, entry 3), the generality of this one-pot protocol was investigated. As summarized in Table 2, the reaction with a series of β-sulfonyl ketones (rac-2a–2f, 2i–2k) bearing electron-rich or electron-deficient substitutes on aromatic ring at R1 or R2 position proceeded smoothly to afford the unreacted sulfones ((R)-2a–2f, 2i–2k) with high enantioselectivityand satisfactory s factors and trisubstituted tetrahydrothiophenes (4a–4f, 4i–4k) were formed in high enantioselectivity and diastereoselectivity. Heteroaryl substrates (rac-2g, 2h, 2l) were well tolerated under the catalytic system. Additionally, the substrates with different functional groups on the sulfone at R3 position (rac-2m–2p)provedtobegoodsubstratesfor this reaction.
|
|
Table 2 Substrate scope.a |
To evaluate the application prospect of this one-pot process, we firstly performed a gram-scale reaction between racemic 2f and 1, 4-dithiane-2, 5-diol (Scheme 2a) to furnish optically pure β-sulfonyl ketones(R)-2f andtetrahydrothiophene 4f. As expected, both products were successfully obtained in the satisfactory yield and stereoselectivity. Furthermore, in order to further facilitate this one-pot enantioselective synthetic process, we decided to start the reaction from α, β-unsaturated enone 5, which was used as the raw material to react with sulfinic acid for the preparation of racemic β-sulfonyl ketone. As shown in Scheme 2b, 5 was quantitatively converted into β-sulfonyl ketone 2f and then transformed into (R)-2f and 4f in untouched yield and enantioselectivity. This process was worked well even in the gram-scale.
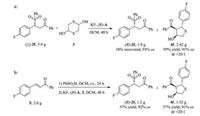
|
Download:
|
| Scheme 2. Gram-scale experiment. | |
In order to gain insight into the reaction pathway, we have carefully studied the reaction process. When the large-scale reaction was performed with substrate 2f, trace α, β-unsaturated enone 5 was observed. This observation indicated that the reaction proceeded with asymmetric β-elimination of β-sulfonyl ketones and subsequent cascade sulfa-Michael/Aldol reaction of the in-situ generated α, β-unsaturated enones. The rate of sulfa-Michael/Aldol reaction was significantly faster than that of the elimination reaction (see Supporting information for details). Based on the experimental observations and previous reports [8, 11], we proposed a plausible pathway of our reaction (Scheme 3).
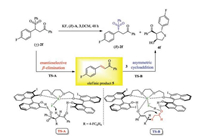
|
Download:
|
| Scheme 3. Plausible reaction pathway. | |
In summary, we developed a one-pot protocol including kinetic resolution of β-sulfonyl ketones and cascade sulfa-Michael/Aldol reaction sequence. According to this method, both unreacted β-sulfonyl ketones and trisubstituted tetrahydrothiophenes were obtained in high enantioselectivities. This process is characterized by the simple operation, mild reaction conditions and high efficiency. Further extension of this protocol is under investigation in our laboratory.
AcknowledgmentsThis study was supported by the Fundamental Research Funds for the Central Universities in China (No. CQDXWL-2014-Z003), the Scientific Research Foundation of China (No. 21402016), Graduate Scientific Research and Innovation Foundation of Chongqing, China (Nos. CYS17044, CYB16032).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.01.047.
| [1] |
(a) S. Doswald, H. Estermann, E. Kupfer, et al., Bioorg. Med. Chem. 2 (1994) 403-410; (b) J. Skarżewski, R. Siedlecka, E. Wojaczyńska, M. Zielińska-Błajet, Tetrahedron: Asymmetry 13 (2002) 2105-2111; (c) M. Teall, P. Oakley, T. Harrison, et al., Bioorg. Med. Chem. Lett. 15 (2005) 2685-2688; (d) F. Reck, F. Zhou, M. Girardot, et al., J. Med. Chem. 48 (2005) 499-506; (e) J. P. Scott, S. F. Oliver, K. M. J. Brands, et al., J. Org. Chem. 71 (2006) 3086-3092; (f) J. P. Scott, D. R. Lieberman, O. M. Beureux, et al., J. Org. Chem. 72 (2007) 4149-4155; (g) M. Nielsen, C. B. Jacobsen, N. Holub, M. W. Paixão, K. A. Jørgensen, Angew. Chem. Int. Ed. 49 (2010) 2668-2679. |
| [2] |
(a) P. Bertus, P. Phansavath, V. Ratovelomanana-Vidal, et al., Tetrahedron Lett. 40 (1999) 3175-3178; (b) P. Mauleón, J. C. Carretero, Chem. Commun. (2005) 4961-4963; (c) H. L. Zhang, X. L. Hou, L. X. Dai, Z. B. Luo, Tetrahedron: Asymmetry 18 (2007) 224-228; (d) T. Llamas, R. G. Arrayás, J. C. Carretero, Angew. Chem. Int. Ed. 46 (2007) 3329-3332; (e) Z. Ding, J. Yang, T. Wang, Z. Shen, Y. Zhang, Chem. Commun. (2009) 571-573; (f) T. Zhou, B. Peters, M. F. Maldonado, T. Govender, P. G. Andersson, J. Am. Chem. Soc. 134 (2012) 13592-13595; (g) B. K. Peters, T. Zhou, J. Rujirawanich, et al., J. Am. Chem. Soc. 136 (2014) 16557-16562. |
| [3] |
(a) K. Hiroi, K. Makino, Chem. Lett. (1986) 617-620; (b) B. M. Trost, M. G. Organ, G. A. O'Doherty, J. Am. Chem. Soc. 117 (1995) 9662-9670; (c) H. J. Gais, T. Jagusch, N. Spalthoff, et al., Chem. -Eur. J. 9 (2003) 4202-4221; (d) M. Ueda, J. F. Hartwig, Org. Lett. 12 (2010) 92-94; (e) X. S. Wu, Y. Chen, M. B. Li, M. G. Zhou, S. K. Tian, J. Am. Chem. Soc. 134 (2012) 14694-14697; (f) X. T. Ma, R. H. Dai, J. Zhang, Y. Gu, S. K. Tian, Adv. Synth. Catal. 356 (2014) 2984-2988. |
| [4] |
(a) I. Shimizu, Y. Ohashi, J. Tsuji, Tetrahedron Lett. 25 (1984) 5183-5186; (b) R. Grigg, Tetrahedron: Asymmetry 6 (1995) 2475-2486; (c) T. Llamas, R. G. Arrayás, J. C. Carretero, Org. Lett. 8 (2006) 1795-1798. |
| [5] |
(a) M. Kennedy, M. A. McKervey, A. R. Maguire, G. H. P. Roos, J. Chem. Soc., Chem. Commun. (1990) 361-362; (b) M. Honma, M. Nakada, Tetrahedron Lett. 44 (2003) 9007-9011; (c) M. Honma, T. Sawada, Y. Fujisawa, et al., J. Am. Chem. Soc. 125 (2003) 2860-2861; (d) M. Takano, A. Umino, M. Nakada, Org. Lett. 6 (2004) 4897-4900; (e) T. Sawada, M. Nakada, Adv. Synth. Catal. 347 (2005) 1527-1532; (f) H. Takeda, H. Watanabe, M. Nakada, Tetrahedron 62 (2006) 8054-8063. |
| [6] |
M.C. Bernabeu, R. Chinchilla, L.R. Falvello, C. Nájera, Tetrahedron:Asymmetry 12 (2001) 1811-1815. DOI:10.1016/S0957-4166(01)00320-2 |
| [7] |
(a) S. Mossé, A. Alexakis, Org. Lett. 7 (2005) 4361-4364; (b) H. Li, J. Song, X. Liu, L. Deng, J. Am. Chem. Soc. 127 (2005) 8948-8949; (c) T. Y. Liu, J. Long, B. J. Li, et al., Org. Biomol. Chem. 4 (2006) 2097-2099; (d) Z. Jin, J. Xu, S. Yang, B. A. Song, Y. R. Chi, Angew. Chem. Int. Ed. 52 (2013) 12354-12358. |
| [8] |
L. Li, Y. Liu, Y. Peng, et al., Angew. Chem. Int. Ed. 55 (2016) 331-335. DOI:10.1002/anie.201508127 |
| [9] |
(a) E. Vedejs, M. Jure, Angew. Chem. Int. Ed. 44 (2005) 3974-4001; (b) L.C. Miller, R. Sarpong, Chem. Soc. Rev. 40 (2011) 4550-4562. |
| [10] |
(a) J. T. Bagdanoff, B. M. Stoltz, Angew. Chem. Int. Ed. 43 (2004) 353-357; (b) A. T. Radosevich, C. Musich, F. D. Toste, J. Am. Chem. Soc. 127 (2005) 1090-1091; (c) C. T. Chen, S. Bettigeri, S. S. Weng, et al., J. Org. Chem. 72 (2007) 8175-8185; (d) A. T. Radosevich, V. S. Chan, H. W. Shih, F. D. Toste, Angew. Chem. Int. Ed. 47 (2008) 3755-3758; (e) D. C. Ebner, R. M. Trend, C. Genet, et al., Angew. Chem. Int. Ed. 47 (2008) 6367-6370; (f) C. T. Chen, J. Q. Kao, S. B. Salunke, Y. H. Lin, Org. Lett. 13 (2011) 26-29; (g) Y. Tan, S. Luo, D. Li, et al., J. Am. Chem. Soc. 139 (2017) 6431-6436. |
| [11] |
M. Duan, Y. Liu, J. Ao, et al., Org. Lett. 19 (2017) 2298-2301. DOI:10.1021/acs.orglett.7b00813 |
| [12] |
P. Zhou, Y. Cai, L. Lin, et al., Adv. Synth. Catal. 357 (2015) 695-700. DOI:10.1002/adsc.v357.4 |
| [13] |
(a) H. Yan, H. B. Jang, J. W. Lee, et al., Angew. Chem. Int. Ed. 49 (2010) 8915-8917; (b) H. Yan, J. Suk Oh, J. W. Lee, C. E. Song, Nat. Commun. 3 (2012) 1212; (c) S. Y. Park, J. W. Lee, C. E. Song, Nat. Commun. 6 (2015) 7512; (d) Y. Liu, J. Ao, S. Paladhi, C. E. Song, H. Yan, J. Am. Chem. Soc. 138 (2016) 16486-16492; (e) L. Yu, X. Wu, M. J. Kim, et al., Adv. Synth. Catal. 359 (2017) 1879-1891; (f) V. Vaithiyanathan, M. J. Kim, Y. Liu, H. Yan, C. E. Song, Chem. -Eur. J. 23 (2017) 1268-1272; (g) S. Paladhi, Y. Liu, B. S. Kumar, et al., Org. Lett. 19 (2017) 3279-3282; (h) M. J. Kim, L. Xue, Y. Liu, et al., Adv. Synth. Catal. 359 (2017) 811-823; (i) S. Y. Park, I. S. Hwang, H. J. Lee, C. E. Song, Nat. Commun. 8 (2017) 14877; (j) S. Paladhi, S. Y. Park, J. W. Yang, C. E. Song, Org. Lett. 19 (2017) 5336-5339. |
 2018, Vol. 29
2018, Vol. 29 




