b Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China
In the past several years, catalytic asymmetric dearomatization (CADA) reactions have attracted enormous research interest due to their powerfulness to construct high valued molecules from readily available aromatic compounds [1]. In many cases, the spirocyclic compounds bearing a quaternary stereogenic center, difficult to be accessed by other methods, could be easily synthesized via CADA reactions. Due to the great potential of the CADA strategy in rapidly building up molecular complexity, various types of arenes are urgent to be further exploited in the CADA reactions. Although CADA reactions of many aromatic compounds have been realized, successful reports are very limited with benzofuran. Among those, asymmetric dearomatization reactions of benzofuran included transition metal catalyzed asymmetric hydrogenation and cycloaddition [2], Lewis acid catalyzed [2, 3]-sigmatropic rearrangement and asymmetric addition reaction [3], organocatalytic cycloaddition [4]. On the other hand, catalytic asymmetric halofunctionalization reactions have witnessed a rapid development recently in that organocatalysts were found to be efficient for promoting halogenation-induced asymmetric functionalization of not only alkenes, but also aromatic compounds [5]. In the other hand, catalytic asymmetric halofunctionalization reactions have witnessed a rapid development recently in that organocatalysts were found to be efficient for promoting halogenation-induced asymmetric functionalization of not only alkenes, but also aromatic compounds. In this regard, our group developed chiral amine-catalyzed asymmetric chlorinative dearomatization reactions of indoles and benzofurans recently [6]. However, the asymmetric bromination dearomatization reactions were limited with only two reports, dearomatization of indoles by Xie, Ma and coworkers using chiral anion phase-transfer strategy and dearomatization of benzofurans by Shi and coworkers using Sc (OTf)3/diphosphine (Scheme 1) [7]. These two reports feature excellent enantioselectivity but employing relatively complex catalytic systems. We envisioned that chiral amine might be suitable catalyst for asymmetric dearomatization bromination of benzofurans. Indeed, brominated spiro[benzofuran-2, 5'-oxazole] compounds could be afforded smoothly from readily available benzofuran derivatives in the presence of chiral tertiary amine catalyst and electrophilic brominating reagent. Herein, we report the results of this study.

|
Download:
|
| Scheme 1. Asymmetric bromination of benzofurans. | |
At the outset, the reaction was evaluated with N-(benzofuran-2-ylmethyl)-4-bromobenzamide (1a) under different reaction conditions (Table 1). The reactions were first conducted with chiral tertial amine (DHQ)2PHAL as an organocatalyst and Nbromophthalimide (NBP) as an electrophilic bromorinating reagent at room temperature (entries 1–5). Among an array of solvents, CF3CH2OH was found to be the optimal one [4g], affording the brominative dearomatized product (2a) bearing spiro [benzofuran-2, 5'-oxazole] backbone in 40% yied with excellent enantioselectivity (96% ee). Next, the brominating reagent was found to have a little influence on the yield, and 54% yield was obtained when 1, 3-dibromo-5, 5-dimethylhydantoin (DBDMH) was used as bromorinating reagent (entries 5–7). The reactions with several other catalysts ((DHQD)2PHAL, (DHQ)2PYR, (DHQD)2PYR, (DHQ)2AQN, (DHQD)2AQN, quinidine and cinchonidine) led to moderate yield and decreased enantiselectivity (entries 8–14). To our delight, decreasing the reaction temperature (-20 ℃), increasing the equivalent of brominative reagent and with N-bromoacetamide (NBAc) as the brominative reagent could enhance the yield of the reaction (entries 15–18). Besides, decreasing the reaction temperature further (-40 ℃) resulted in slightly diminished reaction outcome (entry 19). Therefore, the optimalreaction conditions were determined as shownin entry 18, Table 1: 10 mol% of (DHQ)2PHAL, 1.2 equiv. of NBAc in trifluoroethanol (0.1mol/L) at -20 ℃.
|
|
Table 1 Optimization of the reaction conditions.a |
With the optimal reaction conditions in hand, the substrate scope was then evaluated. Firstly, the substituents on the amide side chain were examined, various electron-withdrawing groups and electron-donating groups were found to be well tolerated, giving the dearomatized products in excellent yields and enantioselectivity (2a–2g, 67%–99% yields, 87%–98% ee). Of particular note, 2-thienyl and olefin substituents were also tolerated (2h–2i, 29%–74% yields), albeit with low yield for 2i. Secondly, the substituents on the benzofuran side were also examined, phenyl, chloride, and fluoride at the different positions of benzofuran could be tolerated, affording the desired dearomatized products in good to excellent yields and enantioselectivity (2j–2m, 88%–99% yields, 86%–89% ee). Finally, the reactions of substrates bearing 5, 7-dichloride on the benzofuran ring or alkyl group on the amide side proceeded smoothly, affording their corresponding products with good yields but moderate enantioselectivity (2n–2o, 74%–86% yields, 21%–34% ee). The low enantioselectivity of 2n is likely due to the steric hindrance of the chloro substituents, however, a full picture is not clear at this stage (Table 2).
|
|
Table 2 Substrate scope.a |
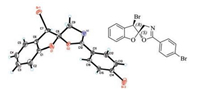
The absolute configuration of 2a was established unambiguously as (2S, 3R) by X-ray crystallographic analysis of an enantiopure sample (Fig. 1). The stereochemistry of other brominative dearomatized products is assigned by analogy. It is suggested that the enantioselectivity determining step is similar with the chlorinative process reported previously by our group [4g].
|
Download:
|
| Fig. 1. Molecular structure of (2S, 3R)-2a. | |
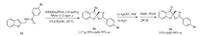
To evaluate the synthetic utility of this methodology, a gramscale reaction of 1a (4mmol) was conducted (Scheme 2). To our delight, product 2a was obtained in 95% yield and 95% ee under standard reaction conditions. Transformation of the Br group in 2a to a ketone group could also be realized over two steps without notable loss of enantioselectivity (51% yield, 94% ee). The resulted spriocyclic compound 3a contains the substructure for biologically active natural products.
|
Download:
|
| Scheme 2. Gram-scale reaction of 1a and transformation of 2a. | |
In conclusion, we have developed a highly efficient bromorination reaction via CADA reaction of benzofuran derivatives under organo-catalytic conditions. In the presence of 10 mol% of (DHQ)2PHAL, a series of chiral brominated spiro[benzofuran-2, 5'-oxazole] derivatives containing two contiguous stereogenic centers were obtained in good to excellent yields and enantioselectivity. The gram-scale reaction and transformation of the product further enhanced the potential utility of this new method. Further investigations on extension of substrate scope and understanding of the reaction mechanism are currently underway.
AcknowledgmentsWe thank the National Key R&D Program of China (No. 2016YFA0202900), National Basic Research Program of China (No. 2015CB856600), the National Natural Science Foundation of China (Nos. 21332009, 21421091), and the Chinese Academy of Sciences (Nos. XDB20000000, QYZDY-SSW-SLH012) for generous financial support.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.01.039.
| [1] |
(a) A. R. Pape, K. P. Kaliappan, E. P. Kündig, Chem. Rev. 100 (2000) 2917-2940; (b) A. Pelter, R. S. Ward, Tetrahedron 57 (2001) 273-282; (c) E. P. Kündig, A. Pape, Top. Organomet. Chem. 7 (2004) 71-94; (d) W. D. Harman, Top. Organomet. Chem. 7 (2004) 95; (e) S. Quideau, L. Pouységu, D. Deffieux, Curr. Org. Chem. 8 (2004) 113-148; (f) F. López Ortiz, M. J. Iglesias, I. Fernández, C. M. Andújar Sánchez, G. R. Gómez, Chem. Rev. 107 (2007) 1580-1691; (g) S. Quideau, L. Pouységu, D. Deffieux, Synlett (2008) 467-495; (h) L. Pouységu, D. Deffieux, S. Quideau, Tetrahedron 66 (2010) 2235-2261; (i) C. X. Zhuo, W. Zhang, S. L. You, Angew. Chem. Int. Ed. 51 (2012) 12662-12686; (j) C. X. Zhuo, C. Zheng, S. L. You, Acc. Chem. Res. 47 (2014) 2558-2573; (k) Q. Ding, X. Zhou, R. Fan, Org. Biomol. Chem. 12 (2014) 4807-4815; (l) W. T. Wu, L. Zhang, S. L. You, Chem. Soc. Rev. 45 (2016) 1570-1580; (m) W. T. Wu, L. Zhang, S. L. You, Acta Chim. Sinica 75 (2017) 419-438; (n) C. Zheng, S. L. You, Chem 1 (2016) 830-857. |
| [2] |
(a) H. M. L. Davies, N. Kong, M. R. Churchill, J. Org. Chem. 63 (1998) 6586-6589; (b) N. Ortega, B. Urban, F. Glorius, Angew. Chem. Int. Ed. 51 (2012) 1710-1713; (c) S. Urban, B. Beiring, N. Ortega, D. Paul, F. Glorius, J. Am. Chem. Soc. 134 (2012) 15241-15244; (d) T. Shibuta, S. Sato, M. Shibuya, et al., Heterocycles 89 (2014) 631-639; (e) H. Kobayashi, Y. Sasano, N. Kanoh, E. Kwon, Y. Iwabuch, Eur. J. Org. Chem. 2016 (2016) 270-273; (f) Q. Cheng, H. J. Zhang, W. J. Yue, S. L. You, Chem 3 (2017) 428-436. |
| [3] |
(a) J. Fu, H. Shang, Z. Wang, et al., Angew. Chem. Int. Ed. 52 (2013) 4198-4202; (b) Q. Tian, J. Bai, B. Chen, G. Zhang, Org. Lett. 18 (2016) 1828-1831. |
| [4] |
(a) N. Dong, X. Li, F. Wang, J. P. Cheng, Org. Lett. 15 (2013) 4896-4899; (b) Y. C. Xiao, C. Z. Yue, P. Q. Chen, Y. C. Chen, Org. Lett. 16 (2014) 3208-3211; (c) B. X. Xiao, W. Du, Y. C. Chen, Adv. Synth. Catal. 359 (2017) 1018-1027; (d) D. Janssen-Müller, M. Fleige, D. Schlüns, et al., ACS Catal. 6 (2016) 5735-5739; (e) Y. Zhang, Y. Guo, Z. Li, Z. Xie, Org. Lett. 18 (2016) 4578-4581; (f) T. Z. Li, C. A. Geng, X. J. Yin, et al., Org. Lett. 19 (2017) 429-431; (g) X. W. Liang, C. Zheng, S. L. You, Adv. Synth. Catal. 358 (2016) 2066-2071. |
| [5] |
(a) A. N. French, S. Bissmire, T. Wirth, Chem. Soc. Rev. 33 (2004) 354-362; (b) G. Chen, S. Ma, Angew. Chem. Int. Ed. 49 (2010) 8306-8308; (c) A. Castellanos, S. P. Fletcher, Chem. -Eur. J. 17 (2011) 5766-5776; (d) C. K. Tan, L. Zhou, Y. Y. Yeung, Synlett (2011) 1335-1339; (e) S. E. Denmark, W. E. Kuester, M. T. Burk, Angew. Chem. Int. Ed. 51 (2012) 10938-10953; (f) U. Hennecke, Chem. Asian J. 7 (2012) 456-465; (g) K. Murai, H. Fujioka, Heterocycles 87 (2013) 763-805; (h) C. K. Tana, Y. Y. Yeung, Chem. Commun. 49 (2013) 7985-7996; (i) J. R. Wolstenhulme, V. Gouverneur, Acc. Chem. Res. 47 (2014) 3560-3570; (j) Y. A. Cheng, W. Z. Yu, Y. Y. Yeung, Org. Biomol. Chem. 12 (2014) 2333-2343; (k) S. Zheng, C. M. Schienebeck, W. Zhang, H. Y. Wang, W. Tang, Asian J. Org. Chem. 3 (2014) 366-376; (l) C. K. Tan, W. Z. Yu, Y. Y. Yeung, Chirality 26 (2014) 328-343; (m) X. W. Liang, C. Zheng, S. L. You, Chem. -Eur. J. 22 (2016) 11918-11933. |
| [6] |
(a) Q. Cai, Q. Yin, S. L. You, Asian J. Org. Chem. 3 (2014) 408-411; (b) Q. Yin, S. L. You, Org. Lett. 15 (2013) 4266-4269; (c) Q. Yin, S. L. You, Org. Lett. 16 (2014) 2426-2429; (d) Q. Yin, S. G. Wang, X. W. Liang, et al., Chem. Sci. 6 (2015) 4179-4183. |
| [7] |
(a) W. Xie, G. Jiang, H. Liu, et al., Angew. Chem. Int. Ed. 52 (2013) 12924-12927; (b) H. Liu, G. Jiang, X. Pan, et al., Org. Lett. 16 (2014) 1908-1911; (c) X. Feng, G. Jiang, Z. Xia, et al., Org. Lett. 17 (2015) 4428-4431; (d) Z. Li, Y. Shi, Org. Lett. 17 (2015) 5752-5755. |
 2018, Vol. 29
2018, Vol. 29