b Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Riyadh, Kingdom of Saudi Arabia
Hydrogels, as an emerging soft matter with high water content, have exhibited a wide range of potentials in biomaterials and biomedicines. Supramolecular hydrogels consisting of short or long peptides are of great research interesting over the past decades, due to the excellent biocompatibility and biofunction of peptides, and liable synthetic procedures of peptide hydrogelators based on well-established solid-phase peptide synthesis (SPPS) protocols [1]. Achieved by rational design of hydrogelators, supramolecular peptide hydrogels are capable of responding to lots of externally applied stimulus [2-6], and the corresponding tunable self-assembly of hydrogelators prompts the hydrogels as "smart" soft materials for potential applications. Pure peptides being made up of diverse amino acids, such as cationic or anionic, aromatic or aliphatic, hydrophobic or hydrophilic, polar or nonpolar amino acids can covalently couple with a variety of molecules, for examples, the aryl moieties (including fluorenylmethoxycarbonyl [7-9], naphthalene [10, 11], and phenothiazine [12]), lipids [13, 14], carbohydrate molecules [15, 16], nucleic acids and nucleobases [17, 18], drugs [19-21], as well as other molecules [22, 23], for novel functional hydrogelators development under custom design. Various intermolecular interactions between hydrogelators, including hydrogen binding, hydrophobic and hydrophilic equilibrium, electrostatic interactions, van der Waals packing or aromatic-aromatic interactions, work cooperatively to drive the self-assembly of hydrogelators, then lead to the hydrogelation. Photosensitive molecules are a kind of exceptional component being utilized to integrate peptides for hydrogelators fabrication, since they can respond to light irradiation with the dynamic changing of hydrogelators structure, then initiate the variation on hydrogels properties. By taking advantages of noncontact and spatiotemporal control, peptide hydrogels bearing photosensitive molecules act as super 'smart' promising materials for a variety of applications. Here, we discussed the development of PPHs including gelation abilities, 'smart' manner and potential applications.
2. Gelation abilities of conjugates consisting of photosensitive molecules and peptidesBy covalently linking different photosensitive moieties with peptides, diverse PPHs including photo-isomerization hydrogels, photo-cage or photo-cleavage hydrogels, and photo-crosslinking hydrogels, have been reported towards the literature work (Fig. 1). Among these hydrogels, photo-cage hydrogels take shape after light irradiation induced photosensitive groups cleavage and release, and photo-crosslinking hydrogels are usually employed for gel mechanical property modulation. Both photo-cage and photo-crosslinking moieties are not contributing to the selfassembly of hydrogelators, while photo-isomerization and photocleavage hydrogels demand the active participation of photoisomerization molecules or photo-cleavage function groups into self-assembly process. Hence, the research about the incorporation of photo-isomerization molecules or photo-cleavage groups into peptide hydrogelators has attracted attentions.

|
Download:
|
| Fig. 1. Simple schematic illustrations of (A) photo-isomerization hydrogel, (B) photo-cleavage hydrogel, (C) photo-cage hydrogel, and (D) photo-crosslinking hydrogel summarized in this review. | |
Our group has ever paid many efforts on photo-isomerization molecules contained peptide hydrogels. Spiropyran/merocyanine (SP/MC) and trans/cis azobenzene (AZO) are two commonly used photo-isomer pairs for peptide hydrogelators development, which the transformation between two isomers is a reversible process upon different light illumination (Fig. 2).

|
Download:
|
| Fig. 2. (A) The reversible transformation between SP isomer and MC isomer upon photo-irradiation and the self-assembly of MC isomers. Reproduced with permission [24]. Copyright 2015, The Royal Society of Chemistry. (B) The reversible transformation between trans/cis AZO upon photo-irradiation. | |
The structural formulas of SP/MC isomer pair are shown in Fig. 2A, MC is a planar structure with a large aromatic group, and rational to be embodied into the structure of hydrogelators, due to the forceful intermolecular aromatic-aromatic interaction between MC molecules. The photo-isomerization of MC to SP interrupts the aromatic-aromatic interaction. For example, our group has reported a smart-designed supramolecular peptide hydrogel based on MC molecule with dual response to light and ligand-receptor interaction [25]. The hydrogelator 1-MC (Fig. 3) contains MC isomer, and a bioactive dipeptide D-Ala-D-Ala which is the ligand of antibiotic vancomycin. MC affords the hydrophobicity and π-π stacking contributing to hydrogel network formation, and the photo-transformation of MC to SP induces the hydrogel disassembling. Alternatively, the binding of dipeptide D-Ala-D-Ala with vancomycin acute disturbs the self-assembly of hydrogelators, also results in the phase transition of gel to solution. Such simple integration of multiple distinct responsive groups or peptides, provides a new strategy for multi-responsive hydrogels development. Afterwards, we systematically investigated the hydrogelation abilities of MC moiety with different peptides, also different linkers between MC and peptides [24]. As the results, MC effectively facilitates the self-assembly of hydrogelators containing miscellaneous peptides, such as various dipeptides, bio-functional peptides containing 3-7 amino acids with different charged and hydrophobic properties. The resulting hydrogels could be degraded once MC moiety is transformed back to SP group by intense visible light illumination.

|
Download:
|
| Fig. 3. Chemical structures of some hydrogelators mentioned in this review. | |
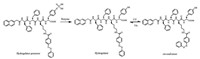
Trans/cis AZO are another regularly used photo-isomers pair, and have served as the building blocks for fabrication of photoisomerization hydrogels too. First and foremost, similar as MC isomer, trans-AZO which exhibits the intermolecular aromaticaromatic interaction is assuredly chosen as the hydrophobic and π-π stacking part for peptide hydrogelators. We have performed the detailed studies on hydrogelation capability of trans-AZO directly integrating short peptide residues including dipeptides, tripeptides and some bioactive short peptides [26]. Aromatic amino acids, such as Phe or Tyr, in the peptide residues, promote the hydrogelation while the cationic amino acids such as Arg and Lys cannot. This result is differing from MC isomer which shows very strong gelation abilities with multiple peptides. We assumed that the much weaker intermolecular aromatic-aromatic interactions between trans-AZO molecules than between MC molecules, causing trans-AZO demand additional synchronous π-π stacking from the peptide residues to achieve the equilibrium of hydrogelation. In addition, trans-AZO substituted some bio-active short peptides also behave as hydrogelators, and one of the hydrogels formed by trans-AZO-Lys-D-Phe-D-Ala (Fig. 3) showed the multiple response to light, heat and addition of vancomycin. Alternatively, Xu's lab have ever incorporated the AZO group at the side chain of a peptide hydrogelator precursor with enzymatic sensitive function group for developing a novel enzyme and light dual-responsive hydrogel [27]. The hydrogelator precursor can be convert to hydrogelator after enzyme treatment, generating a photo-responsive hydrogel as the substrate for light stimuli (Fig. 4). Also, laterally-grafting of AZO units on small peptides act as photo-responsive hydrogelator [28].
|
Download:
|
| Fig. 4. Chemical structures of side chain AZO-modified enzymatic hydrogelator precursor and hydrogelator. | |
The self-assembly of AZO based hydrogel has been characterized too. The hydrogel of trans-AZO conjugating with a dipeptide and an aliphatic C4-trans-AZO-Gly-Gly (Fig. 5A) was revealed the nanostructures as well-defined laminated nanoribbons under transmission electronic microscopy (TEM) (Fig. 5B) [29]. The laminated nanoribbons can transform into short fibers by illuminating the hydrogel by UV light, and recover back to nanoribbons upon visible light irradiation (Fig. 5C).

|
Download:
|
| Fig. 5. (A) Chemical structure of hydrogelator C4-trans-AZO-Gly-Gly. (B) Negative stain TEM image of 5 mmol/L C4-trans-AZO-Gly-Gly hydrogel; (C) cryo-TEM image of 5 mmol/L C4-trans-AZO-Gly-Gly sample illuminated by UV light for 30 min. Reproduced with permission [29]. Copyright 2011, The Royal Society of Chemistry. | |
Photo-cleavage group being included in the hydrogelator does contribution to the self-assembly for hydrogel formation. Light triggered release of the hydrophobic photo-cleavage group from hydrogelator undermines the gelation equilibrium, and induces the hydrogel degradation. Hamachi group has developed a photo-responsive hydrogelator Bhcmoc-Phe-Phe just through the tethering of a simple dipeptide on a bromohydroxycoumarin (Bhc) moiety which is a rather hydrophobic photolabile group with moderate aromatic-aromatic interaction between molecules, and the hydrogelator could be decomposed upon UV light irradiation inducing the gel-sol transition (Fig. 6) [30]. Moreover, Bhc-containing photo-degradation peptide hydrogels allow the efficient cleavage of Bhc group upon near-infrared (NIR) irradiation through two-photon excitation [31, 32].

|
Download:
|
| Fig. 6. Chemical structure of hydrogelator Bhcmoc-Phe-Phe and the photocleavage of Bhc from the hydrogelator. | |
3. Smart behaviors of PPHs
By taking the superiorities of the spatiotemporal controlling, rapid response, as well as the terminable hydrogel response after light source blocking, PPHs behave as super "smart" materials. As we aforementioned, photo-isomerization peptide hydrogels play the role of "smart" materials since they can be achieved the reversible gel-sol phase transformation [33-35], while photocleavage hydrogels are the typical photodegradable hydrogels (Figs. 1A and B).
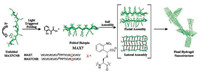
Photo-cage peptide hydrogels expressed as light-activated hydrogelation. The designed peptide hydrogelators loss their self-assembly ability due to the tethering of a photo-cage group, and the gelation can be restored upon light irradiation induced photo-cleavage of the cage group (Fig. 1C). ortho-Nitrobenzyl (o-NB) is a commonly used photolabile group as photo-cage. An example using o-NB to create photo-cage peptide hydrogel was reported by Schneider group. The de novo designed β-hairpin peptide MAX7 can inherently fold and self-assemble to hydrogel in aqueous solution. Covalently linking an o-NB moiety on peptide MAX7 yields peptide MAX7CNB which remains unfolded and unable to self-assemble in aqueous solution due to the electrostatically inhibition from o-NB. Irradiating a certain concentration solution of modified peptide MAX7CNB to generate the uncaged MAX7, triggers the peptide folding and self-assembly, then reinstates the hydrogelation (Fig. 7) [36]. Subsequently, Stupp group also reported the solution-hydrogel transition through the photo-removal of o-NB group on a peptide-lipid amphiphile [37].
|
Download:
|
| Fig. 7. Light-induced hydrogel formation. UV illumination of MAX7CNB results in side-chain decaging and β-hairpin intramolecular folding. Subsequent facial and lateral selfassembly ultimately affords hydrogel material. Reproduced with permission [36]. Copyright 2005, American Chemical Society. | |
Photo-crosslinking peptide hydrogels were utilized for hydrogel mechanical property strengthening. The peptide hydrogelators containing photo-crosslinking groups were able to self-assemble forming physical hydrogels. Upon light illumination, crosslinking reaction between hydrogelators produces hydrogels with hybrid physical-covalent network and thus enhanced mechanical property (Fig. 1D). Still in Schneider group, they reported a photocrosslinking peptide hydrogelator which is a de novo designed β-hairpin peptide equipping photo-crosslinkable dienes [38]. The 22-residue peptide named MLD is capable of folding intramolecularly and self-assembling by increasing the temperature and ionic intensity, to afford β-sheet fibrils causing hydrogelation. By UV irradiation, dienes of non-natural sorbamide derivatives on lysine polymerize along the surface of the gel, which can enhance the gel's material rigidity with a 2.5-fold increase without losing the secondary structure in-situ (Fig. 8). This kind of novel hydrogel allows the manipulation of mechanical properties after the gel has been delivered. Afterwards, same reinforcing of mechanical properties on established hydrogels were achieved by Wang [39] and Parquette [40] groups, respectively using photo induced Ru(bpy)3Cl2-catalyzed tyrosine cross-linking reaction and dimerization of coumarin groups.

|
Download:
|
| Fig. 8. Proposed mechanism of MLD folding and self-assembly leading to hydrogelation and subsequent photopolymerization of its fibrillar network. Reproduced with permission [38]. Copyright 2010, American Chemical Society. | |
4. Applications of PPHs
PPHs acting as super 'smart' materials show practical applications with several examples among the literatures. For photoisomerization hydrogels, one of the MC isomer bearing peptide hydrogel MC1-Arg-Gly-Asp (Fig. 3) showed multiple response to light, pH, heat and metal ion, and behaved as an erasable photolithograph material in our research (Fig. 9A) [24]. The reversible response of the hydrogel to light and heat allowed the photowriting within hydrogel upon visible light irradiation using photomask, and to erase the patterning through a gentle heating/cooling treatment, then generate a new hydrogel for rewriting again. AZO contained hydrogel trans-AZO-Gln-Phe-Ala (Fig. 3) was applied for biomolecule controlled release [26]. As shown in Fig. 9B, biomolecule vitamin B12 could be encapsulated in gel matrix without effecting hydrogelation, and photo-irradiation on the hydrogel greatly accelerated the release of vitamin B12 compare to the one without photo-irradiation. In addition, photo-isomerization peptide hydrogels bearing a new type of photo-switch, arylazopyrazole (AAP), behaved as a light-triggered release platform for multiple payloads including small and large molecules, as well as vesicles, according to a most recent report [41]. Moreover, Hamachi group reported a photoisomerization supramolecular hydrogel based on fumaric double bond with remarkable stiffness for cells biotechnology [42]. The hydrogelator contains three parts which are zwitterionic amino acid lysine, fumaric double bond and two hydrophobic cyclopentyl tails. Achieved by the delicate design, all the interactions such as ion pairing, hydrogen bonding, van der Waals packing and π-π stacking work cooperatively to form hydrogel with very high mechanical strength. The carbon-carbon double bond of fumaric responds to UV light and undergoes a trans to cis transition, then induces the gel degradation due to the break of intermolecular hydrogen bonding. This stiff and photosensitive peptide amphiphile hydrogel is a great platform for two-dimensional (2D) or three-dimensional (3D) spatial patterning, culturing, and differentiation of cells.

|
Download:
|
| Fig. 9. (A) MC1-Arg-Gly-Asp hydrogel is employed as an erasable photolithograph Material. (B) Controlled release of vitamin B12 from hydrogel trans-AZO-Gln-Phe-Ala through UV irradiation compared with spontaneous diffusion. Reproduced with permission [24, 26]. Copyright 2011 and 2015, The Royal Society of Chemistry. | |
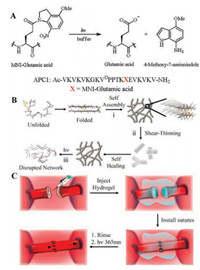
As an example of practical application for photo-cleavage peptide hydrogel, Schneider group reported a peptide hydrogelator which is called anastomosis photocage 1 (APC1) showing the potential to facilitate suturing on anastomosis of micrometre-scale vessels [43]. APC1 containing a protected glutamic acid (4- methoxy-7-nitroindolinyl glutamic acid, MNI-glutamic acid) was designed to be self-folding and self-assembly, leading to the formation of fibrillar network which is composed of a bilayer of β- hairpins through intermolecular hydrogen-bonding. The hydrogelator releases the protecting group under light exposure to introduce a negatively charged glutamate side chain within the hydrophobic bilayer, then results in locally disruptive interaction to initiate the final hydrogel degradation. (Fig. 10B) After syringedelivery, the solid-like hydrogel can be formed at the interspace between vessels, where it can be approximate the ends of micrometre-scale vessels. Then the clamp-free approximation allows the suturing in a normal fashion. The stabilization of fibril network will be destroyed and the hydrogel can be transferred to solution upon light illumination. In this way, the intravascular gel can be eliminated only by washing after the suturing is completed. (Fig. 10C) Besides, the injected gel has promised biocompatibility without gross local inflammation and a typical foreign-body response.
|
Download:
|
| Fig. 10. (A) Photolysis reaction of MNI-glutamate acid producing free glutamate acid, and the peptide sequence of ACP1. (B) Phase transitions of APC1. (ⅰ) APC1 hydrogel formation. (ⅱ) Conversion of the solid-like gel to a viscous gel for syringe delivery. (ⅲ) Final gel-sol phase transition upon irradiation with UV. (C) The potential of ACP1 hydrogel facilitating suturing. Reproduced with permission [43]. Copyright 2016, Macmillan Publishers Limited. | |
In addition, there is no application reported about photo-cage and photo-crosslinking peptide hydrogels to the best of our knowledge. However, the advantages of photo-cage peptide hydrogels and photo-crosslinking peptide hydrogels, which are controllable hydrogel formation and modulable hydrogel mechanic property respectively, could find broad applications in regeneration medicine and tissue engineering.
5. New PPHs based on biaryl-substituted tetrazoleIn addition to the present photo sensitive hydrogels demonstrated above, we have developed a novel photo responsive hydrogel platform as the first example utilizing intramolecular biorthogonal photo-click reaction recently [44]. The hydrogelator consists of a photo-sensitive biaryl-substituted tetrazole motif which provides the hydrophobicity and aromatic-aromatic interaction to integrate with short tri-peptides for hydrogelation, and is capable of undergoing a rapid intermolecular click reaction forming a pyrazoline moiety with instant fluorescence turn on upon mild light illumination (Fig. 11A). The hydrogel prepared from the self-assembly of the hydrogelator thus can be degraded its network matrix by being disturbed the balance between hydrophilic interaction and π-π stacking of the self-assembled system (Fig. 11B). This new type of hydrogels shows great mechanic property at biocompatible concentration level which allows the hydrogels for cell experiments. Such as, the hydrogels display excellent capacities for two dimensional C2C12 cells cultures atop the gels, as well as the 3D human mesenchymal stem cells encapsulation and culture for several days. Moreover, the photosensitivity of the hydrogels allows us to modulate the cellular microenvironments by light in both 2D and 3D cell culture.

|
Download:
|
| Fig. 11. (A) Chemical formula of the intramolecular biorthogonal photo-click reaction based on biaryl-substituted tetrazole. (B) Photo-induced fluorescence turning on and disassembly of hydrogels. Reproduced with permission [44]. Copyright 2013, American Chemical Society. | |
Furthermore, biaryl-substituted tetrazole contained peptide hydrogel with sufficient mechanic property was utilized as the first example of cells-laden peptide gel for miRNA delivery, specific performance is the transportation of miR-122 into encapsulated HepG2 cells for target gene expression repressing [45]. Most recently, we incorporated this miRNA delivery approach with our strategy on intramolecular photo-click reaction induced degradation of hydrogel, to realize the retrieve of encapsulated cells after miRNA regulation. The multiple-functional hydrogelator consists of a photo-sensitive biaryl-substituted tetrazole and a peptide sequence GRGDS, and this custom design facilitates the enhancement of the matrix-cells interaction and nanofibermediated miRNA delivery due to ligand binding of RGD motif with integrin on cells membrane. As a result, the hydrogel encapsulating both U87 cells and miR34a raised the miR34a level in U87 cells after certain time 3D culture, then the U87 cells were collected after photodegradation of hydrogel and alright for further culture in rigid dish [46]. Moreover, we introduced an enzymatic function group phosphate on the tetrazole hydrogelator with intramolecular photo-click reactivity, to create a hydrogelator precursor as the substrate for cell membrane enzyme alkaline phosphatase (ALP). The ALP-instructed dephosphorylation and self-assembly of hydrogelator on cancer cells which overexpresses ALP caused the cell death, then the intramolecular photo-click reaction turned on the fluorescence of self-assembly on cells surface and the induced disassembly of hydrogelator partially restores the cancer cells proliferation [47].
6. Conclusion and prospectiveIn conclusion, we briefly summarized the progress of photosensitive supramolecular peptide hydrogels over the recent decades through the gelation capabilities of different photosensitive molecules integrating with peptides, the 'smart' manner of photosensitive hydrogels response to light stimuli, and the practical applications. In which, our group has contributed lots of work on photo-isomerization peptide hydrogels including both photo-isomers spiropyran and azobenzene. Moreover, a new photosensitive peptide hydrogel platform based on the first example of intramolecular biorthogonal photo-click reaction was presented here, too.
Although lots of supramolecular peptide hydrogels bearing photosensitive molecules have been developed, the practical applications were still lacked for these hydrogels since there were only a few examples among the literatures. Regarding as a practical application with increasing attentions, hydrogels been engineered as promising and versatile scaffolds for cell biotechnology, such as to support the cells adhesion atop, or to encapsulate cells inside their matrix network, mimicking the cellular microenvironment in cell culture, afford a powerful tool for medical applications like bone regeneration [48-51], wound healing [52-54] and tissue engineering [55-58]. However, most of the well-established photosensitive 2D and 3D hydrogel scaffolds for cell research were macromolecule hydrogels, fabrication from polysaccharides, poly PEGs, as well as other natural and synthetic polymers [59, 60]. Several drawbacks limit the popularization of supramolecular peptide hydrogels in cell biotechnology. At first, peptide hydrogels which are physical hydrogels forming from various physical interactions between hydrogelators, show relative weaker mechanic properties than common polymer hydrogels. Then, supramolecular peptide hydrogels are always losing their mass during cell culture due to the sustained dissolve of hydrogelators by culture medium atop of hydrogels, accompanying by the dynamic alteration of gel mechanic properties. In addition, the balance between hydrophilicity and hydrophobicity of peptide amphiphiles in aqueous phase contribute to the formation of supramolecular hydrogels, thence the gel network is sensitive to biomolecule immobilization which may interrupt the gelation equilibrium. However, supramolecular peptide hydrogels allow people to on-demand design and modify the hydrogelators, then to tune the chemical and physical properties of hydrogels, showing much more versatile than high polymer hydrogels. It is always worth to exploit new strategy to overcome these limitations of peptide hydrogels, such as the introducing of reversible covalent linking in peptide hydrogel network to strengthen the gel mechanic properties, or developing the minor influence strategy to graft the biomolecules on hydrogel network, which is the goal for our group to put on efforts currently. Furthermore, development of more novel photosensitive hydrogels bearing different photosensitive moieties as smart materials is always the research interest.
AcknowledgmentsWe would like to acknowledge the financial support from the National Natural Science Foundation of China (Nos. 21572102, 21672103, 21302093). The authors extend their appreciation to the International Scientific Partnership Program ISPP at King Saud University for funding the research work through ISPP#0101.
| [1] |
W.C. Chan, P.D. White, Fmoc Solid Phase Peptide Synthesis:A Practical Approach[M]. New York: Oxford University Press, 2000.
|
| [2] |
Y. Zhao, H. Yokoi, M. Tanaka, T. Kinoshita, T. Tan, Biomacromolecules 9 (2008) 1511-1518. DOI:10.1021/bm701143g |
| [3] |
Z. Yang, G. Liang, B. Xu, Acc. Chem. Res. 41 (2008) 315-326. DOI:10.1021/ar7001914 |
| [4] |
Y. Zhang, H. Gu, Z. Yang, B. Xu, J. Am. Chem. Soc. 125 (2003) 13680-13681. DOI:10.1021/ja036817k |
| [5] |
X. Li, J. Li, Y. Gao, et al., J. Am. Chem. Soc. 132 (2010) 17707-17709. DOI:10.1021/ja109269v |
| [6] |
H. Shigemitsu, T. Fujisaku, S. Onogi, et al., Nat. Protoc. 11 (2016) 1744-1756. DOI:10.1038/nprot.2016.099 |
| [7] |
M. Zhou, A.M. Smith, A.K. Das, et al., Biomaterials 30 (2009) 2523-2530. DOI:10.1016/j.biomaterials.2009.01.010 |
| [8] |
V. Jayawarna, S.M. Richardson, A.R. Hirst, et al., Acta Biomater. 5 (2009) 934-943. DOI:10.1016/j.actbio.2009.01.006 |
| [9] |
H. Geng, Q. Zong, J. You, et al., Sci. China Chem. 59 (2016) 1-10. |
| [10] |
Z. Yang, G. Liang, M. Ma, Y. Gao, B. Xu, J. Mater. Chem. 17 (2007) 850-854. DOI:10.1039/B611255B |
| [11] |
Y. Kuang, B. Xu, Angew. Chem. 52 (2013) 6944-6948. DOI:10.1002/anie.201302658 |
| [12] |
C. Ou, J. Zhang, X. Zhang, Z. Yang, M. Chen, Chem. Commun. 49 (2013) 1853-1855. DOI:10.1039/c3cc38409h |
| [13] |
H. Cui, M.J. Webber, S.I. Stupp, Biopolymers 94 (2010) 1-18. DOI:10.1002/bip.21328 |
| [14] |
M.P. Hendricks, K. Sato, L.C. Palmer, S.I. Stupp, Acc. Chem. Res. 50 (2017) 2440-2448. DOI:10.1021/acs.accounts.7b00297 |
| [15] |
S.W. Liao, T.B. Yu, Z. Guan, J. Am. Chem. Soc. 131 (2009) 17638-17646. DOI:10.1021/ja907097t |
| [16] |
K. Chawla, T.B. Yu, S.W. Liao, Z. Guan, Biomacromolecules 12 (2011) 560-567. DOI:10.1021/bm100980w |
| [17] |
X. Li, Y. Kuang, J. Shi, et al., J. Am. Chem. Soc. 133 (2011) 17513-17518. DOI:10.1021/ja208456k |
| [18] |
D. Wu, J. Zhou, J. Shi, X. Du, B. Xu, Chem. Commun. 50 (2014) 1992-1994. DOI:10.1039/c3cc48946a |
| [19] |
A.G. Cheetham, R.W. Chakroun, W. Ma, H. Cui, Chem. Soc. Rev. 46 (2017) 6638-6663. DOI:10.1039/C7CS00521K |
| [20] |
J. Li, Y. Kuang, Y. Gao, et al., J. Am. Chem. Soc. 135 (2013) 542-545. DOI:10.1021/ja310019x |
| [21] |
Y. Zhou, X. Li, Chin. Chem. Lett. 28 (2017) 1835-1840. DOI:10.1016/j.cclet.2017.04.033 |
| [22] |
X. Du, J. Zhou, J. Shi, B. Xu, Chem. Rev. 115 (2015) 13165-13307. DOI:10.1021/acs.chemrev.5b00299 |
| [23] |
J. Wang, J. Zheng, Y. Cai, et al., Sci. China Chem. 59 (2016) 719-723. DOI:10.1007/s11426-015-5521-8 |
| [24] |
W. Wang, J. Hu, M. Zheng, et al., Org. Biomol. Chem. 13 (2015) 11492-11498. DOI:10.1039/C5OB01912E |
| [25] |
Z. Qiu, H. Yu, J. Li, Y. Wang, Y. Zhang, Chem. Commun. 23 (2009) 3342-3344. |
| [26] |
Y. Huang, Z. Qiu, Y. Xu, et al., Org. Biomol. Chem. 9 (2011) 2149-2155. DOI:10.1039/c0ob01057j |
| [27] |
X. Li, Y. Gao, Y. Kuang, B. Xu, Chem. Commun. 46 (2010) 5364-5366. DOI:10.1039/c0cc00163e |
| [28] |
W. Li, I.S. Park, S.K. Kang, M. Lee, Chem. Commun. 48 (2012) 8796-8798. DOI:10.1039/c2cc34528e |
| [29] |
Y. Lin, Y. Qiao, P. Tang, Z. Li, J. Huang, Soft Matter 7 (2011) 2762-2769. DOI:10.1039/c0sm01050b |
| [30] |
M. Ikeda, T. Tanida, T. Yoshii, I. Hamachi, Adv. Mater. 23 (2011) 2819-2822. DOI:10.1002/adma.201004658 |
| [31] |
C.D.G. Lux, J. Lux, G. Collet, et al., Biomacromolecules 16 (2015) 3286-3296. DOI:10.1021/acs.biomac.5b00950 |
| [32] |
T. Yoshii, M. Ikeda, I. Hamachi, Angew. Chem. 53 (2014) 7264-7267. DOI:10.1002/anie.201404158 |
| [33] |
T.M. Doran, D.M. Ryan, B.L. Nilsson, Polym. Chem. 5 (2013) 241-248. |
| [34] |
J.K. Sahoo, S.K. Nalluri, N. Javid, H. Webb, R.V. Ulijn, Chem. Commun. 50 (2014) 5462-5464. DOI:10.1039/C4CC01431F |
| [35] |
W. Xiong, H. Zhou, C. Zhang, H. Lu, Chin. Chem. Lett. 28 (2017) 2125-2128. DOI:10.1016/j.cclet.2017.09.019 |
| [36] |
L.A. Haines, K. Rajagopal, B. Ozbas, et al., J. Am. Chem. Soc. 127 (2005) 17025-17029. DOI:10.1021/ja054719o |
| [37] |
T. Muraoka, C.Y. Koh, H. Cui, S.I. Stupp, Angew. Chem. 121 (2009) 6060-6063. DOI:10.1002/ange.200901524 |
| [38] |
R.V. Rughani, M.C. Branco, D.J. Pochan, J.P. Schneider, Macromolecules 43 (2010) 7924-7930. DOI:10.1021/ma1014808 |
| [39] |
Y. Ding, Y. Li, M. Qin, Y. Cao, W. Wang, Langmuir 29 (2013) 13299-13306. DOI:10.1021/la4029639 |
| [40] |
S.H. Kim, Y. Sun, J.A. Kaplan, M.W. Grinstaff, J.R. Parquette, New J. Chem. 39 (2015) 3225-3228. DOI:10.1039/C5NJ00038F |
| [41] |
C.W. Chu, B.J. Ravoo, Chem. Commun. 53 (2017) 12450-12453. DOI:10.1039/C7CC07859E |
| [42] |
H. Komatsu, S. Tsukiji, M. Ikeda, I. Hamachi, J. Chem-Asian 6 (2011) 2368-2375. DOI:10.1002/asia.201100134 |
| [43] |
D.J. Smith, G.A. Brat, S.H. Medina, et al., Nat. Nanotechnol. 11 (2016) 95-102. |
| [44] |
M. He, J. Li, S. Tan, R. Wang, Y. Zhang, J. Am. Chem. Soc. 135 (2013) 18718-18721. DOI:10.1021/ja409000b |
| [45] |
J. Li, R. Kooger, M. He, et al., Chem. Commun. 50 (2014) 3722-3724. DOI:10.1039/C4CC00156G |
| [46] |
Z. Zhou, Q. Yi, T. Xia, et al., Org. Biomol. Chem. 15 (2017) 2191-2198. DOI:10.1039/C6OB02667B |
| [47] |
Z. Zhou, X. Xie, Q. Yi, et al., Org. Biomol. Chem. 15 (2017) 6892-6895. DOI:10.1039/C7OB01548H |
| [48] |
J. Kim, I.S. Kim, T.H. Cho, et al., Biomaterials 28 (2007) 1830-1837. DOI:10.1016/j.biomaterials.2006.11.050 |
| [49] |
A. Sanchez-Ferrero, A. Mata, M.A. Mateos-Timoneda, et al., Biomaterials 68 (2015) 42-53. DOI:10.1016/j.biomaterials.2015.07.062 |
| [50] |
A.T. Neffe, B.F. Pierce, G. Tronci, et al., Adv. Mater. (Weinheim Ger.) 27 (2015) 1738-1744. DOI:10.1002/adma.201404787 |
| [51] |
S. Zhang, Y. Guo, Y. Dong, et al., Acs Appl. Mater. Inter. 8 (2016) 13242-13250. DOI:10.1021/acsami.6b01432 |
| [52] |
B. Gupta, R. Agarwal, M.S. Alam, Biomed. Hydrogels (2011), 184-227. |
| [53] |
T. Ito, C. Yoshida, Y. Murakami, Mater. Sci. Eng. C 33 (2013) 3697-3703. DOI:10.1016/j.msec.2013.04.056 |
| [54] |
D.H. Phuc, N.T. Hiep, D.N.P. Chau, et al., Int. J. Polym. Sci. 20146 (2016) 6723716. |
| [55] |
A. Kumar, S.S. Han, Int. J. Polym. Mater. Polym. Biomater. 66 (2017) 159-182. DOI:10.1080/00914037.2016.1190930 |
| [56] |
S. Ahadian, R.B. Sadeghian, S. Salehi, et al., BioconjugateChem. 26 (2015) 1984-2001. |
| [57] |
A. Motealleh, N.S. Kehr, Adv. Healthc. Mater. 6 (2017) 1600938. DOI:10.1002/adhm.v6.1 |
| [58] |
M. Mehrali, A. Thakur, C.P. Pennisi, et al., Adv. Mater. 29 (2017) 1603612. DOI:10.1002/adma.201603612 |
| [59] |
P.M. Kharkar, K.L. Kiick, A.M. Kloxin, Chem. Soc. Rev. 42 (2013) 7335-7372. DOI:10.1039/C3CS60040H |
| [60] |
G. Huang, F. Li, X. Zhao, et al., Chem. Rev. 117 (2017) 12764-12850. DOI:10.1021/acs.chemrev.7b00094 |
 2018, Vol. 29
2018, Vol. 29 


