b State Key Laboratory of Virology, Key Laboratory of Combinatorial Biosynthesis and Drug Discovery(MOE), Wuhan University School of Pharmaceutical Sciences, Wuhan 430071, China;
c Department of Nuclear Medicine, The Second Hospital of Zhejiang University School of Medicine, Hangzhou 310009, China
Molecular imaging techniques are indispensable tools in modern diagnostics, because they will allow highly sensitive and specific measurement of biological processes at the molecular, cellular, tissue and body levels, as well as monitoring of therapeutic responses [1, 2]. To date, a variety of imaging modalities, including positron emission tomography (PET), magnetic resonance imaging (MRI), ultrasound (US) and near infrared fluorescence (NIR) have been actively explored to employ in bioimaging [3-8]. For example, 18F-FDG has been successfully applied as golden standard PET tracer for tumor staging and therapy assessment worldwide, however, the sensitivity and specificity are often less than desired [9]. In order to meet the increasing needs for in vivo basic research and clinical studies, the design of novel functional peptide-based probes with high sensitivity and specificity are still highly demanded. Combinatorial peptide chemistry and phage display technology have profoundly impacted the development of the bioactive synthetic peptides [10-12]. These synthetic peptides could be directly or indirectly labeled with a wide range of imaging modalities via various linkers and conjugated chemistries to construct of peptide-based probes (Fig. 1). In this article, we reviewed the development of peptide-based probes with promising imaging modalities and highlight the successful applications for biomedical imaging in vivo.
|
Download:
|
| Fig. 1. General design of peptide-based probes for biomedical imaging. | |
2. Peptide-based probes for targeted PET imaging
PET is one of the most rapidly growing modalities of biomedical imaging with many successful applications in the preclinical and clinical studies [13]. In general, most low molecular weight peptides have several promising advantages compared with their nanomaterial counterparts, such as favorable pharmacokinetics, tissue distribution and fast clearance from blood [12]. However, considering the decay pathway, half-life and positron energy, 68Ga (half-life, 68 min) and 18F (half-life, 109.8 min) would be better match the pharmacokinetics of most radiopharmaceuticals of peptides [13, 14]. Moreover, the design of a desirable PET peptidebased probe with clinical translation ability is expected to own the following properties: 1) high stability and biocompatibility; 2) high binding affinity and specificity; 3) easy preparation and flexible modification [15]. During the past decade, several types of peptide-based PET probes have widely used to detect various diseases [16-18]. For example, the Arg-Gly-Asp (RGD)-containing peptides labeled with 18F, 68Ga and 64Cu radionuclides to serve as PET probes for integrin αVβ3 targeted imaging of glioblastoma, melanoma, breast and prostate cancer [19-21].
More recently, a small peptide (Arg-Cha-Phe-D)Ser-(D)-ArgTyr-Leu-Trp-Ser, AE105) was developed by combinatorial chemistry and demonstrated high specific binding affinity to urokinase-type plasminogen activator receptor (uPAR) [22, 23]. The uPAR is a glycosylphosphatidylinositol anchored cell membrane receptor that is overexpressed in several types of human cancers including glioblastom, head and neck cancer, prostate cancer [22, 24-25]. Therefore, the ability to non-invasively detect and quantify the uPAR expression of potentially malignant tumors is very attractive for cancer diagnosis. Based on AE105 peptide, several uPAR targeted PET probes have been recently designed and applied to image uPAR expression in vivo [26-28]. For example, radiolabeling of NOTA-AE105 with 68Ga was straightforward, and high image contrast resulting in clear tumor delineation was found for 68Ga-NOTA-AE105 [28]. The absolute tumor uptake was lower for 68Ga-NOTA-AE105 ((0.4 ±0.1)% ID/g) than for 18F-FET ((3.5 ±0.8)% ID/g), however, higher tumor-to-background ratio was obtained for 68Ga-NOTA-AE105 (10.6 ±2.3) than for 18F-FET (1.8 ±0.3) (Fig. 2). In order to optimize the absolute uptake of AE105-based probes in tumor, Mathias et al. modified AE105 with 8-amino octanoic acid (AOC) or polyethylene glycol (PEG) spacers to optimize imaging properties [29]. The result of in vivo PET imaging indicated that introduction of PEG spacers more than doubled the in vivo tumor uptake after 1 h (68Ga-PEG8-AE105, (2.01 ±0.22)% ID/g vs. 68GaAE105, (0.70 ±0.40)% ID/g). Thus, 68Ga-PEG8-AE105 is a promising candidate for human translation for PET imaging of uPAR.

|
Download:
|
| Fig. 2. PET images of glioblastoma tumor (arrows) bearing mice with 18F-FET, and 68Ga-NOTA-AE105. Copied with permission [28]. Copyright 2016, Society of Nuclear Medicine and Molecular Imaging, Inc. | |
3. Fluorescence imaging in the first and second near-infrared (NIR-Ⅱ) window based on peptide-based probes
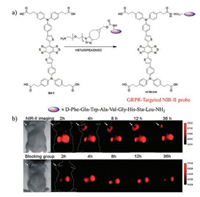
In vivo near-infrared (NIR) fluorescence imaging is an emerging biomedical imaging modality for widely use in both basic research and clinical practice [30]. Actually, NIR imaging has many advantages over other imaging modalities such as its high sensitivity, quick feedback, non-hazardous radiation and low cost, etc. [30-32]. Various NIR-Ⅰ probes with multifunctions are developed maturely such as pH-responsive probes, gold cluster and Glycine-conjugated porphyrin fluorescent probe attached with iRGD [33-37]. For instance, Zhu group designed a mitochondria-targeted fluorescent probe DFB1 on the basis of difluoroboron curcuminoid scaffold to detect cysteine, it displayed a NIR-Ⅰ fluorescence in a Cys concentration environment to monitor endogenous Cys effectively in vivo [36]. In addition, monitoring fluvastatin-stimulated endogenous H2S production through NIR-Ⅰ probes also achieved with high performance [37]. Recent studies also indicated that fluorescence imaging in the second nearinfrared window Ⅱ (NIR-Ⅱ, 1, 000–1, 700 nm) is more desirable than that in NIR-Ⅰ (650–900 nm), owing to the greatly reduced photon scattering and negligible tissue autofluorescence, which can dramatically improve imaging quality and signal-to-noise ratio [38, 39]. To data, a handful of peptide-based probes conjugated to various fluorophores for bioimaging has been reported [40, 41]. For example, the gastrin-releasing peptide receptor (GRPR) has been documented in many human prostate cancer and bombesin (BBN, Glu-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2) peptide exhibits nano-molar affinity and high specificity toward GRPR [42, 43]. Ma et al. reported an Alexa fluor 750 conjugated BBN peptide based NIR-Ⅰ probe, which exhibited high binding specificity to the GRPR on the PC-3 cell membrane [44]. Furthermore, in vivo study showed a significant increase of tumor-to-background ratio in the uptake of this probe at 20 h (7.78:1), as compared to the respective control group. Meanwhile, our group and other groups recently designed a group of NIR-Ⅱ organic dyes based on a donor–acceptor–donor (D-A-D) structure [45-49]. The most promising Q4 dye was conjugated with BBN peptide analogue (RM26 peptide, D-Phe-Gln-Trp-Ala-Val-Gly-HisSta-Leu-NH2) to fabricate the first generation of peptide-based NIR-Ⅱ probes and utilized for GRPR targeted NIR-Ⅱ imaging of prostate cancer in a mouse model (Fig. 3) [45]. Though the RGD peptide based probe has shown promising results for non-invasive molecular imaging of the integrin αVβ3 expression in the NIR-Ⅰ region [50]. Considering the advantages of NIR-Ⅱ imaging, a novel RGD peptide based NIR-Ⅱ probe was developed and represented a highly promising probe for monitoring of early stage glioblastoma in the NIR-Ⅱ window [51].
|
Download:
|
| Fig. 3. (a) Conjugation of Q4-1 with NH2-PEG8-RM26 peptide to prepare a GRPR targeted probe, SCH1100. (b) The NIR-Ⅱ images of PC3 tumor mice (n = 3) at different time points after tail vein injection of SCH1100 with or without blocking agent RM26. Copied with permission [45]. Copyright 2016, Royal Society of Chemistry. | |
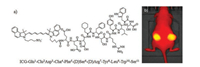
Numerous studies have also indicated that CD13 receptor is an important regulator of endothelial morphogenesis during tumor angiogenesis and overexpression of CD13 is associated with the progression of many tumors, such as prostate cancer and colon cancer [52-54]. The tumor vasculature homing phage containing sequence CNGRCVSGCAGRC was selected by in vivo screening of a phage-displayed peptide library [55]. Therefore, Asn-Gly-Arg (NGR) containing peptide based probes have been developed for CD13-targeted tumor imaging [56, 57]. For example, Chen et al. designed a novel Cy5.5-labeled dimeric NGR peptide probe (Cy5.5- NGR2) via bioorthogonal click chemistry, which exhibited rapid tumor targeting at 0.5 h post-injection (pi) and good tumor-tobackground ratio (2.65 ±0.13) at 2 h pi [58]. These results indicated Cy5.5-NGR2 is a promising probe not only allowing the imaging of CD13 overexpressed tumors, but also having the potential to facilitate monitoring of tumor therapy. Compared with other modalities, NIR imaging is an emerging technique with clear implications for improved cancer surgery by enabling a more distinct delineation of the tumor margins during resection. Recently, Kjaer et al. reported a uPAR-targeted fluorescent probe based on indocyanine green (ICG) conjugated to Glu-Glu-AE105 and the quantitative analysis of the tumor and background uptake showed a tumor to background ratio of 3.52 ±0.17 (Fig. 4) [59]. The results also showed that ICG-AE105 has the potential for clinical translation allowing real-time NIR-imaging in the surgical management of the primary tumor and metastases in pancreatic cancer and other types of uPAR expressing cancers.
|
Download:
|
| Fig. 4. (a) The chemical structure of ICG-Glu-Glu-AE105. (b) NIR images of U87MG tumor bearing mice with ICG-Glu-Glu-AE105. Reproduced with permission [59]. Copyright 2016, Juhl et al. | |
4. Peptide-based probes for PET/NIR dualmodal imaging
A surge in dualmodal instrumentation development for clinical application has sparked the discovery of more dualmodal peptidebased probes to clearly delineate the localization and expression of biochemical markers, and effectively track the tumors with highresolution and high-sensitivity [60]. For instance, a powerful synergy can be achieved by combining PET imaging for localization of tumor in the whole body without penetration limitations, and near infrared fluorescence (NIR) imaging for subsequent accurate delineation of tumor lesions and resection margins [4]. To date, a variety of chemical platforms such as small molecules, nanoparticles and polymers have been widely explored to construct dualmodal probes [61-64]. Of all these dualmodal probes, small peptide-based probes remain promising candidates for clinical applications. However, the preparation of dualmodal peptidebased probes remained a challenging task. Although considerable efforts have been explored to simplify the synthesis of these probes, multiple synthetic procedures are still complicated. Moreover, the extensive use of protection-deprotection procedures and chemoselectivity still heavily hinder the widespread applications of such promising probes in bioimaging [65]. Therefore, developing facile chemical strategy with promising platforms for construction of dualmodal peptide-based probes are still highly demanded.
Based on these challenges, a base-catalyzed chemical strategy with a bicyclo[6.1.0]nonyne (BCN) platform for construction of dualmodal peptide-based probes has been established recently by Sun et al. [65]. Using this strategy, a novel dualmodal PET/NIR-Ⅰ and uPAR-targeted probe 64Cu-CHS1 was prepared and evaluated in U87MG cells and tumor-bearing mice models. The excellent PET/ NIR-Ⅰ imaging characteristics such as good tumor uptake, high tumor to background ratio, and specificity were achieved in the small-animal models (Fig. 5). Meanwhile, Zeng group has described a bifunctional chelator platform for facile construction of dualmodal probes via Cu-free alkyne-azide cycloadditon (SPAAC) chemistry [66]. More importantly, this new platform demonstrated three major advantages, including significantly simplified synthetic steps, ease of purification and higher synthetic yields. More recently, Zeng group has successfully developed a photo-click chemistry strategy for the facile preparation of dualmodal NIR-Ⅰ/PET probes. The diazole photo-click linker between the targeting-ligand and the chelator could also serve as the fluorophore for NIR-Ⅰ imaging [67]. A dualmodal and AE105 peptide based probe was successfully prepared and subsequently applied in the uPAR targeted imaging of the mice bearing U87MG xenograft. Although intraoperative NIR-Ⅰ (650–900 nm) could allow accurate real-time tumor delineation, but the penetration depth of emitted light in biological tissues is limited. There is no doubt that developing novel NIR-Ⅱ dualmodal imaging peptidebased probes for subsequent accurate delineation of tumor lesions and resection margins to achieve more significant improvements in spatial resolution and imaging depth thus has high significance and direct impact on the field of biomedicine [68-70]. In a proofof-concept study, NIR-Ⅱ/PET imaging dualmodality RGD-based probe 68Ga-CHS2 for the first time has been concisely prepared for precise tumor delineation and image-guided surgery (Fig. 6) [71].

|
Download:
|
| Fig. 5. AE105 peptide based and uPAR-targeted probe for NIR/PET dualmodal imaging of U87MG tumor model. Reproduced with permission [65]. Copyright 2015, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | |

|
Download:
|
| Fig. 6. RGD peptide-based and αVβ3-targeted probe for NIR-Ⅱ/PET dual modal imaging of U87MG tumor model. Reproduced with permission [71]. Copyright 2018, Royal Society of Chemistry. | |
5. Outlook
In summary, we have presented and introduced several types of peptide-based probes with respect to their design and applications. As new bioactive peptides and imaging techniques with improved imaging properties continue to be developed, we envision that they will take a prominent role in biomedicine.
AcknowledgmentsThis work was partially supported by grants from the National Natural Science Foundation of China (NSFC Nos. 21708012, 81773674, 81573383, 21390402, 81725009, 21788102, 81425015), 111 Project (No. B17019), NKR & DPC (No. 2016YFA00900), NSFHP (Nos. 2017CFB151, 2017CFA024, 2017CFB711, 2016ACA126), ABRPSTCS (No. SYG201521), NSFJP (No. BK20160387), Shenzhen Science and Technology Research Grant (No. JCYJ20170303170809222), self-determined research funds of CCNU from the colleges, basic research and operation of MOE for the Central Universities (No. 23020205170469), Wuhan Morning Light Plan of Youth Science and Technology (No. 201705304010321).
| [1] |
R. Weissleder, Science 312 (2006) 1168-1171. DOI:10.1126/science.1125949 |
| [2] |
G. Hong, A.L. Antaris, H. Dai, Nat. Biomed. Eng. 1 (2017) 0010. DOI:10.1038/s41551-016-0010 |
| [3] |
J.M. Hoffman, S.S. Gambhir, Radiology 244 (2007) 39-47. DOI:10.1148/radiol.2441060773 |
| [4] |
A. Louie, Chem. Rev. 110 (2010) 3146-3195. DOI:10.1021/cr9003538 |
| [5] |
S. Lee, J. Xie, X. Chen, Chem. Rev. 110 (2010) 3087-3111. DOI:10.1021/cr900361p |
| [6] |
F. Ding, S. Chen, W.S. Zhang, Y.F. Tu, Y. Sun, Bioorg. Med. Chem. 25 (2017) 5179-5184. DOI:10.1016/j.bmc.2017.08.034 |
| [7] |
M.M. Zhang, Y.Q. Wang, H. Shi, et al., ACS Nano 10 (2016) 10075-10085. DOI:10.1021/acsnano.6b05030 |
| [8] |
K.Y. Pu, N. Chattopadhyay, J.H. Rao, J. Control. Release 240 (2016) 312-322. DOI:10.1016/j.jconrel.2016.01.004 |
| [9] |
B. Gulyas, C. Halldin, Q. J. Nucl. Med. Mol. Imaging 56 (2012) 173-190. |
| [10] |
O.H. Aina, R. Liu, J.L. Sutcliffe, et al., Mol. Pharm. 4 (2007) 631-651. DOI:10.1021/mp700073y |
| [11] |
V. Petrenko, Expert. Opin. Drug Deliv. 5 (2008) 825-836. DOI:10.1517/17425247.5.8.825 |
| [12] |
S. Lee, J. Xie, X.Y. Chen, Proc. Biochem. 49 (2010) 1364-1376. DOI:10.1021/bi901135x |
| [13] |
K. Chen, P.S. Conti, Adv. Drug Deliv. Rev. 62 (2010) 1005-1022. DOI:10.1016/j.addr.2010.09.004 |
| [14] |
Y. Sun, X.W. Ma, Z. Zhang, et al., Bioconjug. Chem. 27 (2016) 1857-1864. DOI:10.1021/acs.bioconjchem.6b00279 |
| [15] |
K. Chen, X. Chen, Curr. Top. Med. Chem. 10 (2010) 1227-1236. DOI:10.2174/156802610791384225 |
| [16] |
O.C. Boerman, W.J. Oyen, F.H. Corstens, Semin. Nucl. Med. 30 (2000) 195-208. DOI:10.1053/snuc.2000.7441 |
| [17] |
X.L. Sun, Y. Li, T. Liu, et al., Adv. Drug Deliv. Rev. 110 (2017) 38-51. |
| [18] |
C.F. Ramogida, C. Orvig, Chem. Commun. 49 (2013) 4720-4739. DOI:10.1039/c3cc41554f |
| [19] |
X. Chen, Y. Hou, M. Tohme, et al., J. Nucl. Med. 45 (2004) 1776-1783. |
| [20] |
R. Haubner, H. Wester, W. Weber, et al., Cancer Res. 61 (2001) 1781-1785. |
| [21] |
I. Israel, D. Richter, J. Stritzker, et al., Curr. Cancer Drug Target 14 (2014) 371-379. DOI:10.2174/1568009614666140403123452 |
| [22] |
M. Ploug, S. Østergaard, H. Gardsvoll, et al., Biochemistry 40 (2001) 12157-12168. DOI:10.1021/bi010662g |
| [23] |
R.P. Zhang, Y. Sun, Y. Qiao, J.D. Li, J. Xie, New J. Chem. 39 (2015) 7750-7753. DOI:10.1039/C5NJ01810B |
| [24] |
B. Jacobsen, M. Ploug, Curr. Med. Chem. 15 (2008) 2559-2573. DOI:10.2174/092986708785909012 |
| [25] |
M. Yamamoto, R. Sawaya, S. Mohanam, et al., Cancer Res. 54 (1994) 5016-5020. |
| [26] |
M. Persson, J. Madsen, S. Østergaard, et al., J. Nucl. Med. 53 (2012) 138-145. DOI:10.2967/jnumed.110.083386 |
| [27] |
M. Persson, M. Hosseini, J. Madsen, et al., Theranostics 3 (2013) 618-632. DOI:10.7150/thno.6810 |
| [28] |
M. Persson, M.K. Nedergaard, M. Brandt-Larsen, et al., J. Nucl. Med. 57 (2016) 272-278. DOI:10.2967/jnumed.115.161703 |
| [29] |
D.L. Mathias, Y. Sun, C.H. Liu, et al., Amino Acids 49 (2017) 1089-1100. DOI:10.1007/s00726-017-2407-4 |
| [30] |
S.A. Hilderbrand, R. Weissleder, Curr. Opin. Chem. Biol. 14 (2010) 71-79. DOI:10.1016/j.cbpa.2009.09.029 |
| [31] |
R. Weissleder, M.J. Pittet, Nature 452 (2008) 580-589. DOI:10.1038/nature06917 |
| [32] |
J. Liu, C. Chen, S.L. Ji, et al., Chem. Sci. 8 (2017) 2782-2789. DOI:10.1039/C6SC04384D |
| [33] |
J.R. Hou, D. Jin, B. Chen, et al., Chin. Chem. Lett. 28 (2017) 1681-1687. DOI:10.1016/j.cclet.2017.03.037 |
| [34] |
H.D. Cui, D.H. Hu, J.N. Zhang, et al., Chin. Chem. Lett. 28 (2017) 1391-1398. DOI:10.1016/j.cclet.2016.12.038 |
| [35] |
Q. Zhang, X. Dong, K.P. Wang, et al., Chin. Chem. Lett. 28 (2017) 777-781. DOI:10.1016/j.cclet.2017.03.001 |
| [36] |
P. Zhang, Z.Q. Guo, C.X. Yan, W.H. Zhu, Chin. Chem. Lett. 28 (2017) 1952-1956. DOI:10.1016/j.cclet.2017.08.038 |
| [37] |
L.L. Zhang, H.K. Zhu, C.C. Zhao, X.F. Gu, Chin. Chem. Lett. 28 (2017) 218-221. DOI:10.1016/j.cclet.2016.07.008 |
| [38] |
A.M. Smith, M.C. Mancini, S. Nie, Nat. Nanotechnol. 4 (2009) 710-711. DOI:10.1038/nnano.2009.326 |
| [39] |
G. Hong, J.C. Lee, J.T. Robinson, et al., Nat. Med. 18 (2012) 1841-1846. DOI:10.1038/nm.2995 |
| [40] |
A. Haque, M.S.H. Faizi, J.A. Rather, M. Khan, Med. Bioorg. Chem. 25 (2017) 2017-2034. DOI:10.1016/j.bmc.2017.02.061 |
| [41] |
Y. Sun, S.Y. Hong, X.W. Ma, et al., Chem. Sci. 7 (2016) 5888-5892. DOI:10.1039/C6SC01536K |
| [42] |
P. Mapelli, M. Picchio, Nat. Rev. Urol. 12 (2015) 510-518. DOI:10.1038/nrurol.2015.191 |
| [43] |
A.F. Prasanphanich, P.K. Nanda, T.L. Rold, et al., Proc. Natl. Acad. Sci. U. S. A. 104 (2007) 12462-12476. DOI:10.1073/pnas.0705347104 |
| [44] |
H. Xu, R. Bandari, Z.R. Jiang, et al., J. Nucl. Med.2017 58 (2017) 262. |
| [45] |
Y. Sun, C.R. Qu, H. Chen, et al., Chem. Sci. 7 (2016) 6203-6207. DOI:10.1039/C6SC01561A |
| [46] |
A.L. Antaris, H. Chen, K. Cheng, et al., Nat. Mater. 15 (2016) 235-242. DOI:10.1038/nmat4476 |
| [47] |
K.Q. Shou, C.R. Qu, Y. Sun, et al., Adv. Funct. Mater. 114 (2017) 1700995. |
| [48] |
X.D. Zhang, H.S. Wang, A.L. Antaris, et al., Adv. Mater. 28 (2016) 6872-6879. DOI:10.1002/adma.201600706 |
| [49] |
S.J. Zhu, Q.L. Yang, A.L. Antaris, et al., Proc. Natl. Acad. Sci. U. S. A. 114 (2017) 962-967. DOI:10.1073/pnas.1617990114 |
| [50] |
A. Bunschoten, D.M. van Willigen, T. Buckle, et al., Bioconjug. Chem. 27 (2016) 1253-1258. DOI:10.1021/acs.bioconjchem.6b00093 |
| [51] |
Y. Sun, M.M. Ding, X.D. Zeng, et al., Chem. Sci. 8 (2017) 3489-3493. DOI:10.1039/C7SC00251C |
| [52] |
S.V. Bhagwat, J. Lahdenranta, R. Giordano, et al., Blood 97 (2001) 652-659. DOI:10.1182/blood.V97.3.652 |
| [53] |
J. Teranishi, H. Ishiguro, K. Hoshino, et al., Prostate 68 (2008) 1666-1673. DOI:10.1002/pros.v68:15 |
| [54] |
N. Ikeda, Y. Nakajima, T. Tokuhara, et al., Clin. Cancer Res. 9 (2003) 1503-1508. |
| [55] |
W. Arap, R. Pasqualim, E. Ruoslahti, Science 279 (1998) 377-380. DOI:10.1126/science.279.5349.377 |
| [56] |
A.H. Negussie, J.L. Miller, G. Reddy, et al., J. Control Release 143 (2010) 265-273. DOI:10.1016/j.jconrel.2009.12.031 |
| [57] |
A. von Wallbrunn, J. Waldeck, C. Holtke, et al., J. Biomed. Opt. 13 (2008) 011007. DOI:10.1117/1.2839046 |
| [58] |
G.Q. Li, Y. Xing, J. Wang, P.S. Conti, K. Chen, Amino Acids 46 (2014) 1547-1556. DOI:10.1007/s00726-014-1727-x |
| [59] |
K. Juhl, A. Christensen, M. Persson, M. Ploug, A. Kjaer, PloS One 11 (2016) e0147428. DOI:10.1371/journal.pone.0147428 |
| [60] |
M. Rudin, R. Weissleder, Nat. Rev. Drug Discov. 2 (2003) 123-131. DOI:10.1038/nrd1007 |
| [61] |
S. Mizukami, R. Takikawa, F. Sugihara, M. Shirakawa, K. Kikuchi, Angew. Chem. Int. Ed. 48 (2009) 3641-3643. DOI:10.1002/anie.v48:20 |
| [62] |
Q.L. Fan, K. Cheng, X. Xu, et al., J. Am. Chem. Soc. 136 (2014) 15185-15194. DOI:10.1021/ja505412p |
| [63] |
Y. Sun, S.Y. Hong, X.W. Ma, et al., Chem. Sci. 7 (2016) 5888-5892. DOI:10.1039/C6SC01536K |
| [64] |
Y.Y. Jiang, D. Cui, Y. Fang, et al., Biomaterials 145 (2017) 168-177. DOI:10.1016/j.biomaterials.2017.08.037 |
| [65] |
Y. Sun, X.W. Ma, K. Cheng, et al., Angew. Chem. Int. Ed. 54 (2015) 5981-5984. DOI:10.1002/anie.201500941 |
| [66] |
Y.K. Gai, G.Y. Xiang, X. Ma, et al., Bioconjug. Chem. 27 (2016) 515-520. DOI:10.1021/acs.bioconjchem.6b00034 |
| [67] |
L.Y. Sun, J.L. Ding, W. Xing, et al., Bioconjug. Chem. 27 (2016) 1200-1204. DOI:10.1021/acs.bioconjchem.6b00115 |
| [68] |
G. Hong, S. Diao, J.L. Jun, et al., Nat. Photon. 8 (2014) 723-730. DOI:10.1038/nphoton.2014.166 |
| [69] |
G.S. Hong, S. Diao, A.L. Antaris, H.J. Dai, Chem. Rev. 115 (2015) 10816-10906. DOI:10.1021/acs.chemrev.5b00008 |
| [70] |
C.Y. Li, Y.J. Zhang, M. Wang, et al., Biomaterials 35 (2014) 393-400. DOI:10.1016/j.biomaterials.2013.10.010 |
| [71] |
Y. Sun, X.D. Zeng, Y.L. Xiao, et al., Chem. Sci. 9 (2018) 2092-2097. DOI:10.1039/C7SC04774F |
 2018, Vol. 29
2018, Vol. 29 


