As a potential gasotransmitter [1], SO2 exhibits unique bioactivities such as vasodilation [2]. The abnormal endogenous SO2 level can induce toxicological effects leading to cancer, cardiovascular diseases and neurological disorders [3]. Thus, the monitoring of cellular concentration of it is of great significance. As a readily water soluble compound (40 L SO2 (gas) in 1 L H2O), SO2 forms a very stable hydrated complex (SO2.H2O); Nevertheless, many studies on SO2 fluorescent probes are centered on the detection of bisulfite/sulfite, but not the molecular SO2 (SO2 + H2O ⇌ HSO3- + OH-; HSO3- ⇌ SO32- + H-). In the last few years, fluorescent probes based on nucleophilic addition to aldehydes/ ketones [4, 5] and double bond [6-10] had been developed for its detection.
On the other hand, biothiols, such as cysteine (Cys), homocysteine (Hcy) and glutathione (GSH) are a kind of sulfhydrylcontaining small molecular amino acids that play crucial roles in biological systems. The detection of them are usually performed by the nucleophilicity of the sulfhydryl group [11, 12]. A numerous of reaction-based probes have been developed for either detection or discrimination them [13-17], including probes containing aldehydes. With shorter distance between -SH and -NH2 comparing to GSH, Cys and Hcy can react with aldehydes to form thiazolidines/thiazinanes, which is the most frequently used method to distinguish Cys/Hcy from GSH [18, 19]. Besides, aromatic substitution but not rearrangement is a common used method to differentiate GSH from Cys/Hcy [20-24].
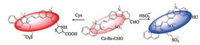
Besides these single site responding probes, dual site probes are emerging recent years. As early as 2013, Guo group [25] designed a three sites chlorinated coumarin-hemicyanine fluorescent probe for selective detection of Cys and GSH from two emission channels. More recently, Yin et al. [26] developed a coumarin-based and two responding sites probe to distinguish Cys and sulfite from different emission channels. More recently, You and their co-wokers also synthesized a two-channel responding probe utilizing malononitrile moiety to detect SO2/ClO- [27]. With the multi-response probe's properties in mind, here we reformed our previously synthesized SO2 detection probe [28], by adding an aldehyde group on the carbazole moiety to selectively recognize SO2 and biothiols. The probe Cz-Bz-CHO could be easily synthesized with the condensation of carbazole and benzoindole (Scheme 1 and Scheme S1 in Supporting information). The fast reaction process makes it possible to real-time image and distinguish the relationship between SO2 and biothiols.
|
Download:
|
| Scheme 1. Proposed sensing mechanism of the dual-site probe | |
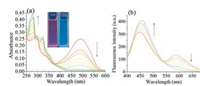
With the probe in hand, the spectral properties of Cz-Bz-CHO with NaHSO3 and three common biothiols were first studied. Interestingly, Cz-Bz-CHO itself possesses two emissions at 445 nm and 590 nm with the excitation at 350/497 nm respectively, and can be used as a two channels imaging probe. When NaHSO3 within 2 equiv. in PBS/DMSO (2/1, v/v, pH 7.4) was added, a gradual increase of fluorescence intensity at 445 nm was observed in less than 4 min (Fig. S2b in Supporting information) and the emission at 590 nm disappeared, with a color change from pale yellow to colorless (Fig. 1b). Accompanying the fluorescence changes, the maximum absorption peak at 497 nm in the UV-vis spectra decreased and the absorption at 295 nm increased (Fig. 1a). With the ratio property in mind (I445/I590), the detection limit was estimated to be 59.9 nmol/L (Fig. S2a in Supporting information).
|
Download:
|
| Fig. 1. UV-vis absorption (a) and fluorescence spectra (b) of 10 μmol/L Cz-Bz-CHO in the presence of 0-30 μmol/L NaHSO3 in PBS/DMSO (2/1, v/v, pH 7.4) system. Inset: Corresponding fluorescent color change under the irradiation of UV lamp (left: probe; right: probe+ NaHSO3). λex = 350 nm. | |
Besides sulfite, Cz-Bz-CHO can also response with biothiols as we designed. The addition of Cys and GSH into a system of Cz-BzCHO in PBS/DMSO (2/1, v/v, pH 7.4) induced a fluorescence enhancement at 590 nm with excitation at 497 nm (Fig. 2b and Fig. S1b in Supporting information). We could also get the consistent outcome from the UV-vis spectra (Fig. 2a, Fig. S1a in Supporting information), in which the absorption peak at 497 nm increased. The reaction can complete within 10 min and be stable even 90 min later, as shown in Figs. S3b and 4b (Supporting information), implying the excellent property of Cz-Bz-CHO in distinguishing sulfite and biothiols. The detection limit of Cz-BzCHO toward Cys and GSH was calculated to be 8.18 μmol/L and 7.22 μmol/L respectively (Figs. S3a and 4a in Supporting information).

|
Download:
|
| Fig. 2. UV-vis absorption (a) and fluorescence spectra (b) of 10 μmol/L Cz-Bz-CHO in the presence of 0-100 μmol/L Cys in PBS/DMSO (2/1, v/v, pH 7.4) system. Inset: Corresponding fluorescent color change under the irradiation of UV lamp (left: probe; right: probe + Cys). λex = 497nm | |
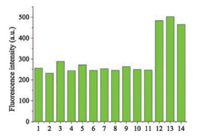
To evaluate the effect of various analytes on Cz-Bz-CHO, Na2S and various amino acids were discussed. As a nucleophile, S2- reacted with aldehyde group first, generating an unstable intermediate product, which induced fluorescence and absorption enhancement at 590 nm and 497 nm respectively (Fig. S5 in Supporting information). After a few minutes, aldehyde group recovered and double bond was attacked, the emission at 590 nm decreased while at 445 nm increased, as shown in Fig. S6 (Supporting information). These phenomenon can be captured by 1H NMR titration experiment (Fig. S7 in Supporting information). When Na2S was added, original aldehyde hydrogen signal at 10.04 ppm splited, indicating that aldehyde was involved in the reaction. One hour later, the aldehyde hydrogen signal recovered. This two-step reaction, which took more than 1 h to be completed, proceeded so slowly, making the interference induced by Na2S negligible (Fig. S8 in Supporting information). Besides, other amino acids also had no interference (Fig. 3).
|
Download:
|
| Fig. 3. The selectivity of 10 mmol/L Cz-Bz-CHO toward various amino acids (10 equiv.). (1) blank, (2) Ala, (3) Val, (4) ASP, (5) Glu, (6) Arg, (7) Lys, (8) Tyr, (9) His, (10) Phe, (11) Ser, (12) Cys, (13) Hcy, (14) GSH. λex = 497nm | |
To verify the proposed mechanism, 1H NMR titration experiments between Cz-Bz-CHO and NaHSO3/Cys/GSH were conducted. As shown in Fig. S9 (Supporting information), in a mixture of DMSO-d6 and D2O (4:1, v/v) solution, the proton signals at 8.68 and 7.83 ppm, which belong to the double bound disappeared after the addition of NaHSO3 and new signals appeared at 5.19 and 3.76 ppm. However, aldehyde hydrogen signal persisted throughout. In contrast, Cys induced the disappearance of aldehyde hydrogen signal at 10.04 ppm and the appearance of new resonances centered at 5.68 and 5.57 ppm, which were assigned to the methane protons of the thiazolidine diastereomers [29]. Although GSH caused the same fluorescence enhancement with Cys, we did not know the exact mechanism currently.
Considering the capability of the probe to distinguish NaHSO3 and biothiols under physiological conditions (pH 7.4), the potential application of Cz-Bz-CHO for fluorescence imaging of NaHSO3 and biothiols in living cells was explored. HeLa cells were previously treated with NEM (N-ethylmaleimide, a common intracellular thiol scavenger), then Cz-Bz-CHO for NaHSO3 imaging was conducted. As shown in Fig. 4, fluorescence from the blue channel was only seen. When incubated with probe without previous NEM treatment, fluorescence from the origin channel revealed and blue channel grew brighter, indicating potential relationships between reactive sulfur species. These results indicated the application of the probe for cellar imaging of NaHSO3 and biothiols from blue channel and red channel respectively.

|
Download:
|
| Fig. 4. Confocal images of Cz-Bz-CHO towards biothiols and sulfite. (a) Fluorescence image of HeLa cells pre-treated with NEM (0.5 mmol/L) for 20 min, and incubated with Cz-Bz-CHO (5 μmol/L) for 10 min; (b) Fluorescence image of (a) from the blue channel; (c) Overlay of (a) and (b); (d) Bright field of (a). (e) Fluorescence image of HeLa cells incubated with Cz-Bz-CHO (5 μmol/L) for 10 min from the orange channel; (f) Fluorescence image of (e) from the blue channel; (g) Overlay of (e) and (f); (h) Bright field of (e). Excited at 488 nm for the orange channel (550-680 nm); Excited at 405 nm for the blue channel (420-500 nm). Scale bar: 20 μm (a-d), 10μm (e-h). | |
In summary, we have developed a dual-site fluorescent probe for the selective detecting of NaHSO3 and biothiols based on the different fluorescent responses caused by different active site reaction. NaHSO3 reacts with conjugate bond specifically, while Cys responses with aldehyde. The probe displays excellent selectivity and sensitivity, and could be applied in imaging of biothiols in live cells successfully. We anticipate the current probe can provide some useful information for the further probes design and analytes detection.
AcknowledgmentThis work was financially supported by the National Natural Science Foundation of China (Nos. 21572147, 21232005 and J1103315).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.11.011.
| [1] |
(a) D. Liu, Y. Huang, D. Bu, et al., Cell Death Dis. 5(2014) e1251; (b) X. B. Wang, H. F. Jin, C. S. Tang, J. B. Du, Clin. Exp. Pharmacol. Physiol. 37(2010) 745-752. |
| [2] |
(a) Z. H. Meng, H. F. Zhang, Inhalation Toxicol. 19(2007) 979; (b) Z. H. Meng, H. Geng, J. L. Bai, G. G. Yan, Inhalation Toxicol. 15(2003) 951. |
| [3] |
(a) G. K. Li, N. Sang, Ecotoxicol. Environ. Saf. 72(2009) 236-241; (b) J. L. Li, R. J. Li, Z. Q. Meng, Eur. J. Pharmacol. 645(2010) 143-150; (c) P. J. Thompson, H. Vally, Thorax 56(2001) 763-769; (d) N. Sang, Y. Yun, H. Y. Li, et al., Toxicol. Sci. 114(2010) 226-236. |
| [4] |
C. Yin, X. Li, Y. Yue, et al., Sens. Actuators B 246 (2017) 615-622. DOI:10.1016/j.snb.2017.02.127 |
| [5] |
H. Agarwalla, S. Pal, A. Paul, et al., J. Mater. Chem. 4 (2016) 7888-7894. DOI:10.1039/C6TB02637K |
| [6] |
Y. Zhang, L. Guan, H. Yu, et al., Anal. Chem. 88 (2016) 4426-4431. DOI:10.1021/acs.analchem.6b00061 |
| [7] |
J. Yang, K. Li, J.T. Hou, et al., ACS Sens. 1 (2016) 166-172. DOI:10.1021/acssensors.5b00165 |
| [8] |
D.P. Li, Z.Y. Wang, H. Su, J.Y. Miao, B.X. Zhao, Chem. Commun. 53 (2017) 577-580. DOI:10.1039/C6CC06459K |
| [9] |
Y. Zhu, W. Du, M. Zhang, et al., J. Mater. Chem. 5 (2017) 3862-3869. DOI:10.1039/C7TB00726D |
| [10] |
D.P. Li, Z.Y. Wang, J. Cui, et al., Sci. Rep. 7 (2017) 45294. DOI:10.1038/srep45294 |
| [11] |
L.Y. Niu, Y.Z. Chen, H.R. Zheng, et al., Chem. Soc. Rev. 44 (2015) 6143-6160. DOI:10.1039/C5CS00152H |
| [12] |
X. Wang, Z.B. Zeng, J.H. Jiang, Y.T. Chang, L. Yuan, Angew. Chem. Int. Ed. 55 (2016) 13658-13699. DOI:10.1002/anie.201510721 |
| [13] |
Y. Liu, X. Lv, M. Hou, Y. Shi, W. Guo, Anal. Chem. 87 (2015) 11475-11483. DOI:10.1021/acs.analchem.5b03286 |
| [14] |
H. Tong, J. Zhao, X. Li, et al., Chem. Commun. 53 (2017) 3583-3586. DOI:10.1039/C6CC09336A |
| [15] |
K. Liu, H. Shang, X. Kong, W. Lin, J. Mater. Chem. B 5 (2017) 3836-3841. DOI:10.1039/C7TB00187H |
| [16] |
Z. Liu, X. Zhou, Y. Miao, et al., Angew. Chem. Int. Ed. 56 (2017) 5812-5816. DOI:10.1002/anie.201702114 |
| [17] |
M. Zheng, H. Huang, M. Zhou, et al., Chem. Eur. J. 21 (2015) 10506-10512. DOI:10.1002/chem.201500885 |
| [18] |
J.Y. Xie, C.Y. Li, Y.F. Li, et al., Anal. Chem. 88 (2016) 9746-9752. DOI:10.1021/acs.analchem.6b02646 |
| [19] |
Z. Yang, N. Zhao, Y. Sun, et al., Chem. Commun. 48 (2012) 3442-3444. DOI:10.1039/c2cc00093h |
| [20] |
M.Y. Jia, L.Y. Niu, Y. Zhang, et al., ACS Appl. Mater. Interfaces 7 (2015) 5907-5914. DOI:10.1021/acsami.5b00122 |
| [21] |
L. He, Q. Xu, Y. Liu, et al., ACS Appl. Mater. Interfaces 7 (2015) 12809-12813. DOI:10.1021/acsami.5b01934 |
| [22] |
X.F. Yang, Q. Huang, Y. Zhong, et al., Chem. Sci. 5 (2014) 2177-2183. DOI:10.1039/c4sc00308j |
| [23] |
H.J. Xiang, H.P. Tham, M.D. Nguyen, et al., Chem. Commun. 53 (2017) 5220-5223. DOI:10.1039/C7CC01814B |
| [24] |
L. Song, L.M. Ma, Q. Sun, et al., Chin. Chem. Lett. 27 (2016) 330-334. DOI:10.1016/j.cclet.2015.12.012 |
| [25] |
J. Liu, Y.Q. Sun, Y. Huo, et al., J. Am. Chem. Soc. 136 (2014) 574-577. DOI:10.1021/ja409578w |
| [26] |
Y. Yue, F. Huo, P. Ning, et al., J. Am. Chem. Soc. 139 (2017) 3181-3185. DOI:10.1021/jacs.6b12845 |
| [27] |
K. Dou, Q. Fu, G. Chen, et al., Biomaterials 133 (2017) 82-93. DOI:10.1016/j.biomaterials.2017.04.024 |
| [28] |
Y. Liu, K. Li, K.X. Xie, et al., Chem. Commun. 52 (2016) 3430-3433. DOI:10.1039/C5CC10505F |
| [29] |
O. Rusin, N.N.S. Luce, R.A. Agbaria, et al., J. Am. Chem. Soc. 126 (2004) 438-439. DOI:10.1021/ja036297t |
 2018, Vol. 29
2018, Vol. 29 


