b bCollaborative Innovation Center of Chemical Science and Engineering(Tianjin), Nankai University, Tianjin 300071, China
In recent years, stimuli-responsive assemblies attracted more and more attention due to their prospective applications in the fields of chemistry, biomaterial, and biomedical [1-6]. Among them, supramolecular assemblies have the superiorities of simple construction, controllable assembling behavior, and tunable response to various external stimuli including enzyme, pH, light, temperature or other factors [7-14]. Recently, a variety of charged macrocyclic hosts, including cyclodextrins [15-17], pillararenes [18-20], calixarenes [21, 22] and so on, have been widely reported able to construct supramolecular assemblies through electrostatic interactions with guests and lowered the critical aggregation concentration (CAC), and supramolecular assemblies with pH-responsive properties can provide a new pathway to design effective carriers of biologically important matter [23-26]. Hruby et al. reported an efficient pH-sensitive nanocontainer employing Eudragit L100-55 and nonionic surfactant Brij98 to realize the loading of trypsin [27]. Wang et al. reported a pH-responsive supramolecular vesicle based on water-soluble pillar[6]arene, showing the loading and rapid release of mitoxantrone [28]. Lim et al. reported a targeted drug delivery system based on p-phosphonated calix[4]arene vesicles to achieve the release of paclitaxel under acidic environments [29]. Up to now, most of these researches were focused on the quick and targeted release of drugs or biofunctional molecules [30], but supramolecular assemblies as sustained release carriers were still rare. Scherman et al. reported a system that achieved the sustained release of proteins from self-assembled supramolecular polymer hydrogel, which is important for sustained therapeutic applications [31]. Herein, we developed a pH-responsive and sustained release supramolecular system for biofunctional molecule adenosine 5'-triphosphate (ATP) based on two biocompatible components, i.e., sulfato-β-cyclodextrin (SCD) and polyethylenimine (PEI). It was well documented that cyclodextrins (CDs) possess the characteristics of good water solubility and biocompatibility, convenient preparation and so on [32, 33], and CDs can include various guest molecules in their hydrophobic cavity with selectivity [34, 35]. On the other hand, PEI is a kind of polycations that has been widely used for the gene delivery [36, 37]. Because the polycationic structure of PEI could induce the supramolecular aggeration of polyanionic SCD, a new supramolecular assembly PEI/SCD was successfully constructed. Although there is no host-guest interaction between SCD and PEI, the electrostatic interactions between anionic SCD and cationic PEI may promote the formation of the nanoparticles. In addition, because the protonation degree of PEI is different under different pH values, the nanoparticles showed a good pH-responsive assembly/ disassembly behavior and the loading/sustained release abilities towards ATP (Scheme 1).

|
Download:
|
| Scheme 1. Schematic illustration of PEI/SCD supramolecular nanoparticle | |
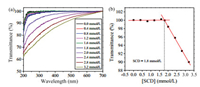
As a polycationic polymer, PEI is unable to form a self-assembly system independently due to its high water solubility.Interestingly, a simple mixture of SCD and PEI in aqueous solution showed the clear Tyndall effect, indicating the existence of large aggregates in solution. No Tyndall effects could be observed in the cases of free SCD or PEI in the control experiments, revealing that neither free SCD nor PEI can form aggregates under the same conditions (Fig. S1 in Supporting information). The critical aggregation concentration (CAC) of SCD in the presence of PEI (5μg/mL) was measured by monitoring the dependence of optical transmittance changes with increasing SCD concentration in aqueous solution. In the presence of PEI, the optical transmittance of SCD initially unchanged and then gradually decreased with increasing SCD concentration as a result of the formation of aggregates in solution (Fig. 1a), and the PEI-induced CAC value of SCD was measured as 1.8 μmol/L according to the plot of optical transmittance at 450 nm versus the concentration of SCD (Fig. 1b). In the control experiments, the optical transmittance of SCD showed no appreciable changes in the absence of PEI (Fig. S2 in Supporting information).
|
Download:
|
| Fig. 1. (a) Optical transmittance of SCD solutions at different concentrations in the presence of PEI (5 μg/mL) at 25 ℃. (b) Dependence of optical transmittance at 450 nm on SCD concentration in the presence of PEI (5 μg/mL). | |
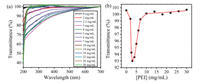
Moreover, the optical transmittance spectra and the plot of optical transmittance at 450 nm by gradually adding PEI to the solution of SCD at a fixed concentration of 2.5 μmol/L were performed to measure the preferable mixing ratio between SCD and PEI. As shown in Fig. 2, the optical transmittance of resultant mixture at 450 nm rapidly decreased and then gradually increased thereafter to a quasi-plateau, and a minimum was observed at a concentration of PEI at 3 μg/mL. The rapid decrease of optical transmittance indicated the formation of large aggregates between SCD and PEI. Subsequently, the further addition of excess PEI led to the decrease of turbidity because of the disassembly of aggregate. Therefore the preferable mixing ratio of PEI/SCD assembly was determined as 3 μg/mL PEI/2.5 μmol/L SCD. The control experiments showed that the optical transmittance of PEI presented no appreciable changes in the absence of SCD (Fig. S2 in Supporting information).
|
Download:
|
| Fig. 2. (a) Optical transmittance of SCD (2.5 μmol/L) by increasing the concentration of PEI from 0 to 30 μg/mL at 25 ℃. (b) Dependence of the optical transmittance at 450 nm on PEI concentration in the presence of SCD (2.5 μmol/L). | |
Furthermore, the PEI/SCD assembly was characterized by dynamic light scattering (DLS), zeta potential and transmission electron microscopy (TEM). The DLS results gave an average hydrodynamic diameter of PEI/SCD assembly as ca. 135 nm, accompanied by a relatively narrow size distribution (Fig. 3a), and free SCD or PEI showed no DLS signals under the same concentrations. Moreover, TEM image of PEI/SCD assembly showed a number of spherical particles with a diameter of ca. 90-160 nm, which was basically consistent with the DLS results. A possible rationale for the layer-by-layer structure may be as follow: Free SCD molecules could not form the large self-aggregate. Upon the addition of PEI, one PEI and several SCD would form a complex. Subsequently, various complexes integrated together to form the large layer-by-layer aggregate that curved to generate layer-bylayer spheres with alternating shell structure. The resulting nanoparticles were simultaneously stabilized by electrostatic interactions. Zeta potential experiments gave a relatively high positive zeta potential of PEI/SCD assembly as + 20.01 mV (Fig. S3 in Supporting information), indicating that the assembly may have the satisfactory capability of associating anionic substrates. In addition, the stability of assembly was also determined. At the room temperature, the optical transmittance, turbidity and Tyndall effect of PEI/SCD assembly showed no appreciable changes for at least 6 h (Fig. S4 in Supporting information). On the other hand, the optical transmittance of PEI/SCD assembly could keep constant with gradually increasing the temperature from 10 ℃ to 70 ℃, accompanied by the invariability of turbidity and Tyndall effect (Fig. S5 in Supporting information). These results jointly demonstrated the good stability of PEI/SCD assembly.

|
Download:
|
| Fig. 3. (a) DLS data, (b) TEM image of PEI/SCD assembly. [SCD] = 2.5μmol/L, [PEI] = 3μg/mL | |
Interestingly, pH was found able to control the assembly/ disassembly behavior of PEI/SCD nanoparticles. The pH value of PEI/SCD assembly in aqueous solution was 6.3. With increasing the pH value of solution from 6.3 to 11.4, the optical transmittance of PEI/SCD assembly at 450 nm gradually enhanced, accompanied by the disappearance of Tyndall effect, demonstrating the disassembly of large aggregates. Thereafter, the enhanced optical transmittance at 450 nm almost recovered to the original level with decreasing the pH from 11.4 to 6.3, accompanied by the re-appearance of Tyndall effect, indicating the re-generation of PEI/SCD assembly (Figs and b). Significantly, these cycles could be repeated for tens of times (Figs. 4c and d), indicating a good pH responsiveness of PEI/SCD assembly.

|
Download:
|
| Fig. 4. (a) Optical transmittance and (b) dependence of the optical transmittance at 450 nm of the PEI/SCD assembly with increasing the pH from 6.3 to 11.4 gradually and then decreasing the pH from 11.4 to 6.3 gradually. (c) Optical transmittance and (d) dependence of the optical transmittance at 450 nm of the PEI/SCD assembly with varying the pH between 6.3 and 11.4. [SCD] = 2.5 μmol/L, [PEI] = 3 μg/mL. | |
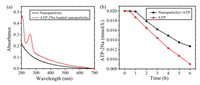
Possessing a large positive zeta potential, the PEI/SCD assembly may have a possibility of loading substrates with negative charge. Herein, adenosine 50-triphosphate disodium salt (ATP-2Na) was selected as an anionic model substrate to be loaded onto the PEI/ SCD assembly inspired by the electrostatic interactions between the cationic assembly and the phosphate chains of adenosine triphosphate (ATP) with negative charge [38]. After loading ATP- 2Na, an obvious absorption peak at 259 nm appeared in the UV-vis spectrum of PEI/SCD assembly (Fig. 5a), and therefore the encapsulating and loading ratio of ATP-2Na were calculated as 63.53% and 44.10%, respectively. The stability experiments showed that the nanoparticles were stable for at least 6 h after loading ATP (Fig. S7 in Supporting information). In addition, the sustained release of ATP-2Na from the PEI/SCD assembly was also investigated. As seen in Fig. 5b, ATP-2Na loading on the PEI/SCD assembly exhibited a slower release than that of free ATP-2Na, showing a sustained and smooth release of ATP-2Na within 12 h at physiological pH (pH 6.3 and 7.3, Figs. S8a and S8b in Supporting information).
|
Download:
|
| Fig. 5. (a) Absorption spectra of ATP-2Na-loaded PEI/SCD assembly and nanoparticle alone. (b) Sustained release of ATP-2Na in the presence or absence of PEI/SCD assembly within 6 h in the solution of pH 6.3. | |
In conclusion, we successfully constructed a kind of supramolecular nanoparticles based on sulfato-β-cyclodextrin and polyethylenimine. The resulting nanoparticles could change the assembly and disassembly states by controlling pH. The zeta potential of pHresponsive supramolecular nanoparticles is positive, which enables its potential application as the delivery carrier of ATP.
AcknowledgmentWe thank the National Natural Science Foundation of China (Nos. 21432004, 21672113, 21772099 and 91527301) for financial support.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.12.022.
| [1] |
X. Zhang, S. Rehm, M.M. Safont-Sempere, F. Würthner, Nat. Chem. 1 (2009) 623-629. DOI:10.1038/nchem.368 |
| [2] |
C.J.F. Rijcken, O. Soga, W.E. Hennink, C.F. van Nostrum, J. Control. Release 120 (2007) 131. DOI:10.1016/j.jconrel.2007.03.023 |
| [3] |
van Hest J.C.M., Nature 461 (2009) 45-47. DOI:10.1038/461045a |
| [4] |
Y. Zhu, J. Shi, W. Shen, et al., Angew. Chem. Int. Ed. 44 (2005) 5083. DOI:10.1002/(ISSN)1521-3773 |
| [5] |
Y. Zhou, H. Li, Y.W. Yang, Chin. Chem. Lett. 26 (2015) 825-828. DOI:10.1016/j.cclet.2015.01.038 |
| [6] |
X. Ma, Y. Zhao, Chem. Rev. 115 (2015) 7794-7839. DOI:10.1021/cr500392w |
| [7] |
M. Liong, J. Lu, M. Kovochich, et al., ACS Nano 2 (2008) 889-896. DOI:10.1021/nn800072t |
| [8] |
Y. Wang, N. Ma, Z. Wang, X. Zhang, Angew. Chem. Int. Ed. 46 (2007) 2823-2826. DOI:10.1002/(ISSN)1521-3773 |
| [9] |
H.B. Cheng, H.Y. Zhang, Y. Liu, J. Am. Chem. Soc. 135 (2013) 10190-10193. DOI:10.1021/ja4018804 |
| [10] |
X.Y. Hu, Y. Chen, Y. Liu, Chin. Chem. Lett. 26 (2015) 862-866. DOI:10.1016/j.cclet.2015.01.003 |
| [11] |
D.S. Guo, K. Wang, Y.X. Wang, Y. Liu, J. Am. Chem. Soc. 134 (2012) 10244-10250. DOI:10.1021/ja303280r |
| [12] |
Q. Zhao, Y. Chen, Y. Liu, Chin. Chem. Lett. 29 (2018) 84-86. DOI:10.1016/j.cclet.2017.07.024 |
| [13] |
S. Sun, J.B. Shi, Y.P. Dong, et al., Chin. Chem. Lett. 24 (2013) 987-992. DOI:10.1016/j.cclet.2013.07.014 |
| [14] |
X. Wu, L. Gao, J. Sun, X.Y. Hu, L. Wang, Chin. Chem. Lett. 27 (2016) 1655-1660. DOI:10.1016/j.cclet.2016.05.004 |
| [15] |
R.J. Shi, Y. Chen, X.F. Hou, Y. Liu, RSC Adv. 6 (2016) 15175-15179. DOI:10.1039/C5RA28043E |
| [16] |
D. Zhao, Y. Chen, Y. Liu, Chin. Chem. Lett. 26 (2015) 829-833. DOI:10.1016/j.cclet.2014.11.028 |
| [17] |
X.F. Hou, Y. Chen, Y. Liu, Soft Matter 11 (2015) 2488-2493. DOI:10.1039/C4SM02896A |
| [18] |
Y. Cao, X.Y. Hu, Y. Li, et al., J. Am. Chem. Soc. 136 (2014) 10762-10769. DOI:10.1021/ja505344t |
| [19] |
K. Jie, Y. Zhou, Y. Yao, B. Shi, F. Huang, J. Am. Chem. Soc. 137 (2015) 10472-10475. DOI:10.1021/jacs.5b05960 |
| [20] |
Y. Chang, K. Yang, P. Wei, et al., Angew. Chem. Int. Ed. 53 (2014) 13126-13130. DOI:10.1002/anie.201407272 |
| [21] |
N. Basilio, Martín-Pastor M., García-Río L., Langmuir 28 (2012) 6561-6568. DOI:10.1021/la3006794 |
| [22] |
K. Wang, D.S. Guo, M.Y. Zhao, Y. Liu, Chem. Eur. J. 22 (2016) 1475-1483. DOI:10.1002/chem.201303963 |
| [23] |
S. Angelos, Y.W. Yang, K. Patel, J.F. Stoddart, J.I. Zink, Angew. Chem. Int. Ed. 47 (2008) 2222-2226. DOI:10.1002/(ISSN)1521-3773 |
| [24] |
H. He, S. Chen, J. Zhou, et al., Biomaterials 34 (2013) 5344-5358. DOI:10.1016/j.biomaterials.2013.03.068 |
| [25] |
X.Y. Hu, K. Jia, Y. Cao, et al., Chem. Eur. J. 21 (2015) 1208-1220. DOI:10.1002/chem.201405095 |
| [26] |
X. Wu, L. Gao, X.Y. Hu, L. Wang, Chem. Rec. 16 (2016) 1216-1227. DOI:10.1002/tcr.201500265 |
| [27] |
A. Riabtseva, L.I. Kaberov, J. Kucka, et al., Langmuir 33 (2017) 7649-772. DOI:10.1021/acs.langmuir.7b01072 |
| [28] |
Q. Duan, Y. Cao, Y. Li, et al., J. Am Chem. Soc. 135 (2013) 10542-10549. DOI:10.1021/ja405014r |
| [29] |
J. Mo, P.K. Eggers, Z. Yuan, C.L. Raston, L.Y. Lim, Sci. Rep. 6 (2016) 23489. DOI:10.1038/srep23489 |
| [30] |
J. Tian, C. Yao, W.L. Yang, et al., Chin. Chem. Lett. 28 (2017) 798-806. DOI:10.1016/j.cclet.2017.01.010 |
| [31] |
E.A. Appel, X.J. Loh, S.T. Jones, C.A. Dreiss, O.A. Scherman, Biomaterials 33 (2012) 4646-4652. DOI:10.1016/j.biomaterials.2012.02.030 |
| [32] |
K. Uekama, F. Hirayama, T. Irie, Chem. Rev. 98 (1998) 2045-2076. DOI:10.1021/cr970025p |
| [33] |
J.J. Li, Y. Chen, J. Yu, N. Cheng, Y. Liu, Adv. Mater. 27 (2017) 1701905. |
| [34] |
Y. Chen, Y.M. Zhang, Y. Liu, Chem. Commun. 46 (2010) 5622-5633. DOI:10.1039/c0cc00690d |
| [35] |
Y. Chen, Y. Liu, Chem. Soc. Rev. 39 (2010) 495-505. DOI:10.1039/B816354P |
| [36] |
W.T. Godbe, K.K. Wu, A.G. Mikos, Proc. Natl. Acad. Sci. U. S. A. 96 (1999) 5177-5181. DOI:10.1073/pnas.96.9.5177 |
| [37] |
Y. He, G. Cheng, L. Xie, et al., Biomaterials 34 (2013) 1235-1245. DOI:10.1016/j.biomaterials.2012.09.049 |
| [38] |
H.Y. Chen, M. Zhao, J.H. Tan, et al., Tetrahedron 70 (2014) 2378-2382. DOI:10.1016/j.tet.2014.02.041 |
 2018, Vol. 29
2018, Vol. 29 


