b College of Natural and Health Sciences, Zayed University, Abu Dhabi 144534, United Arab Emirates
Irregularities in use of prohibited additives emerge constantly despite of repeated prohibition in China. Chrysoidin, mainly used in dyeing leather, textiles and wood products, is a kind of cationic dye with high tinting strength and good lightfastness. In virtue of its price superiority against natural pigments, chrysoidin is often added into food and beverage, causing serious impact on consumers' health. By skin contact, breath in or mistakenly ingestion, it can cause chronic poisoning [1, 2]. Chinese food additives hygiene standards have not included it as a permitted food additive because of its teratogenic and carcinogenic behavior [3]. Many methods are used to achieve the identification and quantification of chrysoidin, such as thin layer chromatography (TLC) [4], high-performance liquid chromatography (HPLC) [5] and mass spectrography [6]. Despite of the good performance these methods showed, expensive equipment and laborious operation are always required. Thus it would be of significant meaning to develop a simple and rapid method for chrysoidin detection.
Malachite green serves as industrial raw material and also has special effects in the prophylaxis and treatment for saprolegniasis. Since 1933, it has been widely used in aquaculture as an effective parasiticide and bactericide in many countries [7]. During the 1990s, researches had disclosed many negative side effects of malachite green including high toxicity/residual, and carcinogenicity. For these horrible setbacks, Chinese Ministry of Agriculture has listed it as a banned substance in aquaculture. Yet illegal usages repeatedly emerge despite of prohibitions, mainly due to the lack of inexpensive specific medicine for saprolegniasis. In Henan and Hubei Provinces, fishmonger add malachite green into the water to keep fishes in good health condition, fishes with broken scales can last longer during transportation. At present, mostly used detection methods for malachite green are HPLC [8] and liquid chromatography tandem mass spectrometry (LC-MS) [9]. With the same reason to that of chrysoidin, it is necessary to develop a simple and rapid method for the detection of malachite green.
Surface-enhanced Raman spectroscopy (SERS), as a sensitive, nondestructive and rapid detection method, has raised great interest and been widely applied in the detections of illegal additives [10]. For successful SERS measurements, a substrate with good performance is the most essential factor. In our previous works, SERS substrates were fabricated based on glycidyl methacrylate-ethylene dimethacrylate (GMA-EDMA) porous material and silanized diatomaceous support, and applied in detection for several pesticides and real samples [11].
In China, food and beverage security problems emerge one after another not only because illegal additives are profitable compared with lawful ones, but also concerns to a weak supervision. For monitoring the illegal additives and harmful substances, many methods such as HPLC showed setbacks of complexity, timeconsuming, limited operation in the laboratory and high price. SERS is a potential method for banned compound analysis, however limited by the lack of cheap and effective substrates. Traditional SERS method of using gold colloid as substrate suffers from low sensitivity that cannot satisfy measuring requirements. Uncontrollable integration and bad repeatability makes it unsuitable for quantitative determination. Tedious preparing processes and a short shelf time limit its application in routine laboratory analysis. When it comes to determination of chrysoidin and malachite green in practice, the detection method should be simple, cost effective, sensitive and stable. The silanized support based SERS substrate developed by our group has shown good sensitivity and repeatability. Based on commercialized material, this kind of substrates have good uniformity and offers sensitive rapid detection [12]. Combined with the portable Raman instrument, our optimized SERS method could be a promising candidate for inexpensive quick detection of chrysoidin and malachite green.
This work mainly focuses on determinations of malachite green in aquatic water and chrysoidin in beverage with silanized support based SERS substrate. Satisfactory experiment results suggested that the SERS substrate can be an ideal tool for detection of harmful substances.
Chloroauric acid tetrahydrate (greater than 47.8% purity (gold)) was obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Sodium citrate (greater than 99% purity), 201 red silanized diatomaceous support (similar to Chromosorb PAWHDMS, John-Manville Co., Ltd. New York, America) was purchased from Shanghai First Reagent Industry (Shanghai, China). 4-Mercaptopyridine was bought from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Chrysoidin and malachite green were obtained from Aladdin-Reagent Co., Ltd. (Shanghai, China). Ethylenediamine was gotten from Shanghai Lingfeng Chemical Reagent Co., Ltd. (Shanghai, China). Beverage of Sprite was bought from a local supermarket. Aquatic water was obtained from a native farmer's market. All solutions were prepared with Milli-Q water (18 MΩ cm).
To modify amidogen on surface of the 201 red silanized diatomaceous support, 15 mg support material was weighted and mixed with 1 mol/L aqueous solution of ethanediamine in a conical flask. The flask was then placed in a shaking bath at 70 ℃ for 5 h. After the amination process was done, redundant solution was removed by several times of wash by ultra-pure water. At this point, the amidogen modified diatomaceous support was stored in water for further use.
Gold colloid were synthesized by the G. Frens method of reducing chloroauric acid tetrahydrate with sodium citrate as reductant [13, 14]. 50 mL 1 mmol/L chloroauric acid tetrahydrate was boiled in a 250 mL round-bottom flask. Upon boiling, 1.85 mL 1% (w/v) sodium citrate aqueous solution was added into the solution and the solution was left to boil for an additional 15 min with mild stirring. When the solution turned wine red, the reduction was completed and the gold colloid was stored in a reagent bottle. 15 mg amidogen modified diatomaceous support was added with 3 mL gold colloid, after shaking on a vibrator until red color of the gold colloid faded, gold nanoparticles were completely assembled onto the material surface. Finally, the material was washed with ultra-pure water to remove the AuNPs which had not been immobilized. Thus gold nanoparticle functionalized diatomaceous support was prepared and kept under water for SERS detection.
Stock solution of 4-mercaptopyridine (4-Mpy) at a concentration of 1 mmol/L was prepared. Solutions of chrysoidin and malachite green with a concentration of 10 mg/L were prepared. Solutions at different concentrations were obtained by diluting the stock solutions with ultrapure water.
Beverage sample containing chrysoidin was prepared by adding 100μL chrysoidin standard solution into 900μL Sprite. The sample was passed through a self-made polyamide column for absorption of the pigment. And 2 mL ultra-pure water was injected through the column to remove other additives. Then the absorbed chrysoidin was eluted with 100μL acetonitrile for two times, eluant was diluted with same volume of water and taken for SERS measurement.
To simulate a sample of aquatic water with malachite green, 100μL standard solution of malachite green was mixed with 900μL aquatic water collected from fishmongers, and was directly taken for SERS measurement without further treatment.
A few particles of above mentioned SERS substrates were moved into a 2 mL centrifuge tube, mixed with 1 mL sample solution. After several minutes shaking on a vibrator, the materials were taken out and put on a quartz plate for SERS detection. SERS spectra were recorded using a portable Raman instrument (i-Raman, Bwtek Inc., U.S.A.) attached with a microscope (20 × objective). The laser source wavelength was 785 nm and the integration time was 10 s.
Stability and repeatability of the SERS substrate is of great significance for practical applications. In our previous works, based on material of silanized diatomaceous support, amidogen modification and gold nanoparticles decoration have been carried out to prepare a SERS substrate, which has showed wonderful performance in detections of triazophos and phosmet. In the current work, we investigate effect of particle size of diatomaceous support on SERS activity for a further optimization. Diatomaceous support in different mesh numbers of 40-60, 60-80 and 80-100 were used to prepare SERS substrates, and 1 ×10-9 mol/L 4-Mpy was used as a probe molecule to test their SERS activity. When the particle size was 80-100 meshes, the substrate is too small to cover the laser spot during the detection, thus very unsteady and weak signals were obtained. And when mesh number was 40-60, substrate particles are oversized that suffer from poor mechanical strength, damage were constantly caused during usage or transformation, result in no or weak signal that was often unstable. The moderate size of SERS substrate showed better SERS activity towards 4-Mpy molecules, and good repeatability. For better SERS signal intensity, loading quantity of sample was appropriately raised from 200μL to 1 mL, which is not too much liquid for substrate particles to disperse uniformly in the sample solution. Compared to our previous works, the substrate's detection limit toward 4-Mpy has reached 1 × 10-9 mol/L after optimization. To conclude, the mesh number of diatomaceous support was chosen to be 60-80, and loading volume of sample was decided to be 1 mL.
Silanized support based substrate has been proved to remain its SERS activity for more than 6 months in our previous work [12], which guarantees the stability of this detection method. The substrate is produced in large batches and its usage is simple thus suitable for routine laboratory analysis. Furthermore, our method showed good sensitivity, so we applied it into analysis of illegal additives.
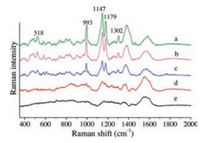
Different concentrations of chrysoidin aqueous solution were analyzed under optimized SERS detection condition, the SERS spectrums were shown in Fig. 1. For chrysoidin, peak at 518 cm-1 is attributed to skeletal deformity of the molecule. Peak at 993 cm-1 derived from the C-C bending. C-N stretching contributed to the peak at 1147 cm-1 [3]. From the figure, these characteristic peaks due to chrysoidin can be observed clearly when its concentration was 0.01 mg/L or more. Even when the concentration was lower to 0.001 mg/L, peaks at 993 cm-1 and 1147 cm-1 can still be found. For comparing, SERS spectra of chrysoidin detected with traditional method of using Au colloid as substrate were also recorded and the results suggested that clear SERS signals of chrysoidin can only be observed when concentration of chrysoidin was higher than 0.5 mg/L. Meng et al. developed a novel gold nanodumbbells SERS substrate and used it for detection of chrysoidin in the orange juice and coke and the detection limit was 5 mg/L [15]. Xie et al. designed a SiO2@Au nanoshells SERS substrate and limit of detection for chrysoidine was 0.5 mg/L [3]. Compared with works of some other groups, lower detection limit was reached by our method.
|
Download:
|
| Fig. 1. SERS spectra of chrysoidin with different concentrations of (a) 1 mg/L, (b) 0.1 mg/L, (c) 0.01 mg/L, (d) 0.001 mg/L, and (e) blank signal collected from the SERS substrate. | |
For real sample of Sprite, as described in the Experimental section, polyamide was used to absorb chrysoidinwhen the sample passed through, while other additives such as natural pigment and carbohydrate were washed away with ultra-pure water in the impurities eluting process. Acetonitrile was selected as the elution solvent for desorption of chrysoidin. The eluant was diluted with water, and was measured to collect SERS spectrum according to method described above. As shown in Fig. 2, characteristic peaks of chrysoidin at 993, 1147, 1179 cm-1 can still be clearly observed at concentration of 0.01 mg/L, which seemed to be found with a little in signal of 0.001 mg/L and of course completely disappeared in blank signal. Although some peaks due to the experiment system can be observed, after a closer look none of them interfered with finger print peaks of chrysoidin. According to the National standard of China (GB/T 23496-2009), limit of detection for chrysoidin in beverage was 0.01 mg/kg which is equal to the detectability of our method. However, compared with HPLC method used in National standard, our method is much more rapid, simple and inexpensive, indicating its potential in rapid monitoring of poisonous and harmful substances in food and drinks.

|
Download:
|
| Fig. 2. SERS spectra of chrysoidin in Sprite with different concentrations of (a) 10 mg/L, (b) 1 mg/L, (c) 0.1 mg/L, (d) 0.01 mg/L, (e) 0.001 mg/L and (f) blank signal collected from the SERS substrate. | |
In order to achieve quantitative measurement of chrysoidin, and to test the stability and detection repeatability of the developed SERS substrate, various concentrations of chrysoidin's aqueous solution have been collected using the SERS substrate and the concentration calibration curve plotted by the peak height of the 993 cm-1 is shown in Fig. 3. It was found that there is a good linear relationship between the Raman intensities and chrysoidin concentration in the concentration range of 0.001-0.05 mg/L. Each data point represents the average value from 6 SERS spectra and the error bars show the standard deviations. A good linearity and the slight error have proved our method to be a stable and precise way for quick on-site detection.

|
Download:
|
| Fig. 3. Linear correlation of Raman intensity at 993 cm-1 with the concentration changing from 0.001-0.05 mg/L. | |
To further investigate the capability of the SERS substrate, we applied it to the detections of malachite green aqueous solution. As malachite green exists in aqueous solution in practice, a series of concentrations were prepared by adding standard solution of malachite green into aquatic water. All solutions were directly taken for SERS measurements with method described above. It was interesting to find that the lowest detectable concentration for malachite green reached 0.0001 mg/L with our method, which showed much better sensitivity compared with traditional method of using gold colloid as substrate, with which 0.1 mg/L malachite green can hardly be detected. Sun et al. developed a SERS film that can detect 0.036 mg/L malachite green on fish skin [16]. Xiao et al. prepared silver film over nanospheres and minimum detection limit for MG was down to 0.004 mg/L [17]. Our method showed better sensitivity and simplicity compared with these works. For SERS spectra of malachite green, peak at 436, 1170, 1360 and 1613 cm-1 were caused by out of-plane modes of phenyl-C-phenyl, in-plane modes of ring C-H bending, N-phenyl stretching, ring C-C stretching, respectively [18]. Out-of-plane modes of ring C-H, C-H rocking and N-phenyl stretching contributed to peaks at 795, 1215 and 1393 cm-1 respectively [19]. Peak at 914 cm-1 derived from C-H out of-plane bending [20].
Illegal usage for malachite green is mainly by adding it into aquatic water, thus it would be meaningful that we achieve determination of malachite green in water, since it is a banned substance in aquaculture industry. So we collected some water from different fishpond raising different kinds of fishes including perch, mandarin fish and snakehead. These kinds of aquatic water were used to prepare solutions for malachite green in different concentrations, and the solutions were measured with the SERS substrate developed in this work. As shown in Fig. 4, characteristic peaks of malachite green at 436, 1170, 1360, 1393, 1613 cm-1 are still clear to be seen at 0.0001 mg/L, which disappeared at a lower concentration, suggesting the detection limit for malachite green in fish pond water reached 0.0001 mg/L. This experimental phenomenon came out same in all three kinds of fish raising water. Our SERS method showed convenience and good sensitivity for rapid judgment of adding malachite green into fish-raising water, which could be helpful to control abusive usage for malachite green in aquaculture.

|
Download:
|
| Fig. 4. SERS spectra of malachite green with different concentrations of (a) 0.01 mg/L, (b) 0.001 mg/L, (c) 0.0001 mg/L in perch raising water, (d) 0.0001 mg/L in mandarin fish raising water, (e) 0.0001 mg/L in snakehead raising water, (f) 0.00001 mg/L and (g) blank signal collected from the SERS substrate. | |
In this work, we further improved the operating conditions of silanized support based SERS substrate and applied this rapid simple sensitive method into the detection for chrysoidin and malachite green. Detection limits for chrysoidin and malachite green in aqueous solutions reached 0.001 mg/L and 00001 mg/L respectively. To focuses on illegal acts of adding malachite green into aquatic water and adding chrysoidin into beverage to fake orange/lemon flavor, we simulated some real samples and performed detailed testing. When concentration of chrysoidin in Sprite was 0.01 mg/L and malachite green in fish pond water reached 0.0001 mg/L, clear SERS signals could be collected from the substrate. Compared with other testing methods, our method showed advantage in time spending, test costing and sensitivity. This indicates that our SERS method could have good potential in quick monitoring of toxic and harmful substance.
AcknowledgmentThis work was supported by Science and Technology Commission of Shanghai Municipality (No.17142202600).
| [1] |
C.C. Wang, L.C. Juang, T.C. Hsu, et al., J. Colloid Interface Sci. 273 (2004) 80-86. DOI:10.1016/j.jcis.2003.12.028 |
| [2] |
S. Wang, Y. Boyjoo, A. Choueib, Z.H. Zhu, Water Res. 39 (2005) 129-138. DOI:10.1016/j.watres.2004.09.011 |
| [3] |
Y. Xie, T. Chen, Y. Cheng, et al., Spectrochim. Acta A 132 (2014) 355-360. DOI:10.1016/j.saa.2014.04.096 |
| [4] |
F. Soponar, A.C. Moţ, C. Sârbu, J. Chromatogr. A 1188 (2008) 295-300. DOI:10.1016/j.chroma.2008.02.077 |
| [5] |
T. Reyns, S. Fraselle, D. Laza, Van Loco J., Biomed. Chromatogr. 24 (2010) 982-989. |
| [6] |
W.J. Gui, Y. Xu, L.F. Shou, G.N. Zhu, Y.P. Ren, Food Chem. 122 (2010) 1230-1234. DOI:10.1016/j.foodchem.2010.02.054 |
| [7] |
S. Srivastava, R. Sinha, D. Roy, Aquat. Toxicol. 66 (2004) 319-329. DOI:10.1016/j.aquatox.2003.09.008 |
| [8] |
J. Zmudzki, J. Chromatogr. A 1089 (2005) 187-192. DOI:10.1016/j.chroma.2005.07.004 |
| [9] |
P. Scherpenisse, A.A. Bergwerff, Anal. Chim. Acta 529 (2005) 173-177. DOI:10.1016/j.aca.2004.08.009 |
| [10] |
Y. Zhang, Y. Huang, F. Zhai, et al., Food Chem. 135 (2012) 845-850. DOI:10.1016/j.foodchem.2012.04.082 |
| [11] |
H. Zhang, Y. Kang, P. Liu, et al., Anal. Lett. 49 (2016) 2268-2278. DOI:10.1080/00032719.2016.1147577 |
| [12] |
H. Zhang, L. Sun, Y. Zhang, et al., Talanta 174 (2017) 301-306. DOI:10.1016/j.talanta.2017.06.025 |
| [13] |
G. Frens, Colloid. Polym. Sci. 250 (1972) 736-741. |
| [14] |
G. Frens, Nat. (Phys. Sci.) 241 (1973) 20-22. DOI:10.1038/physci241020a0 |
| [15] |
J. Meng, S. Qin, L. Zhang, L. Yang, Appl. Surf. Sci. 366 (2016) 181-186. DOI:10.1016/j.apsusc.2016.01.078 |
| [16] |
H. Sun, H. Liu, Y. Wu, Mater. Lett. 207 (2017) 125-128. DOI:10.1016/j.matlet.2017.07.064 |
| [17] |
G.N. Xiao, W.B. Huang, Z.H. Li, Plasmonics 12 (2017) 1169-1175. DOI:10.1007/s11468-016-0372-5 |
| [18] |
G.H. Gu, J.S. Suh, J. Raman Spectrosc. 41 (2009) 624-627. DOI:10.1002/jrs.v41:6 |
| [19] |
L. He, N.J. Kim, H. Li, Z. Hu, M. Lin, J. Agric. Food. Chem. 56 (2008) 9843-9847. DOI:10.1021/jf801969v |
| [20] |
K. Lai, Y. Zhang, R. Du, et al., J. Food. Meas. Charact. 5 (2011) 19-24. |
 2018, Vol. 29
2018, Vol. 29 


