Supramolecular polymers constructed by single non-covalent interaction have got extensive attention and many achievements have been made in this field since Jean-Marie Lehn's report in 1990 [1]. Thus, more and more attention has been paid to constructing supramolecular polymers based on multiple non-covalent interactions owing to their numerous interesting properties and potential applications [2-5]. These non-covalent interactions include hydrogen bonding [6], metal coordination [7], host-guest recognition [8], aromatic stacking [9], and so forth. Therein, the host-guest association has been widely applied for its good selectivity and convenient stimuli responsiveness to these polymers [10-13], and the metal-ligand interactions endow metal supramolecular polymers (MSPs) strong coordination binding abilities and some properties in electromagnetic aspects [14-16]. Among various building blocks for the construction of this type of supramolecular polymers, coumarin (Cou) and terpyridine (TP) are regarded as two of the most powerful units. The inherent advantages of using coumarin and terpyridine can not only form the steady 1:2 inclusion complexes with γ-cyclodextrin (γ-CD) but also realize photodimerization [17]. The strong binding between terpyridine and metal ions (Zn2+) enables the supramolecular polymers through intermolecular coordination to realize selfassembly [18, 19].
Following the above idea, we design and synthesize the compound Cou-BP-TP (Scheme 1), which contains coumarin unit and terpyridine unit as two terminals, and linked with bipyridine unit. Furthermore, benefiting from host-guest association with γ-CD and metal-ligand interaction with Zn2+, a linear ternary supramolecular polymer system based on Cou-BP-TP/γ-CD/ZnCl2 via non-covalent bind was successfully constructed in aqueous phase. The fibrous morphology of supramolecular polymer was found by transmission electron microscope (TEM) results.

|
Download:
|
| Scheme 1. Chemical structures of compounds 1 to 5 and schematic illustration for the construction of the supramolecular polymer p-Cou-BP-TP via multiple non-covalent interactions. | |
The particular experimental section was shown in Supporting information. Compounds 1-4 were synthesized according to the previous reports. And the compound 5 was firstly synthesized. Compound 2 (675 mg, 1.3 mmol, 2 equiv.) and compound 4 (300 mg, 0.65 mmol, 1 equiv.) were dissolved in N, N-dimethylformamide (30 mL). The reaction mixture was heated under reflux for 72 h. After cooling down to room temperature, the suspension was filtrated and deep brown solid was collected with dryness (94 mg, yield 7%). All the target molecules were characterized by proton nuclear magnetic resonance (1H NMR) to confirm the structure. Cou-BP-TP was firstly synthesized. 1H NMR spectra, 13C NMR spectra and electronic spray ionization (ESI) high-resolution mass spectra shown in Supporting information could confirm the structure of Cou-BP-TP.
In supramolecular system, the formation of host-guest inclusion complexes depends on the size match of host molecule and guest molecule. γ-CD with a cavity diameter of 0.85 nm can form 1:2 inclusion complexes with Cou unit [17]. The formation of supramolecular polymer between Cou-BP-TP and γ-CD was explicitly stated by the 1H NMR spectrum (Fig. 1) and 2D ROESY NMR spectrum experiments in D2O (Fig. S8 in Supporting information). As we can see from Fig. 1, the NMR signal of Ha, Hb, Hc, Hd and He shifted upfield by 0.03 ppm, 0.13 ppm, 0.11 ppm, 0.22 ppm, 0.20 ppm, respectively, owing to the inclusion interaction of γ-CD. Furthermore, the nuclear overhauser effect (NOE) signals between Ha-He of Cou-BP-TP and protons of γ-CD also proved the formation of supramolecular polymer conclusively in Fig. S8. Diffusion-ordered spectroscopy (DOSY) further validated the formation of supramolecular assemblies. The complexed γ-CD and Cou-BP-TP diffuse together with a main peak of diffusion rate of 1.76×10-10 m2/s (Fig. S9 in Supporting information), which is much slower than that of free Cou-BP-TP (2.10×10-10 m2/s at 8 mmol/L, Fig. S10 in Supporting information) and γ-CD (3.10×10-10 m2/s at 4 mmol/L, Fig. S11 in Supporting information).

|
Download:
|
| Fig. 1. Partial 1H NMR spectrum of (a) Cou-BP-TP, (b) Cou-BP-TP: γ-CD = 2:1 in D2O at 25 ℃, both concentrations were 8.0 mmol/L | |
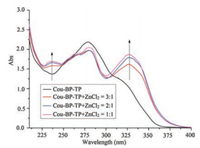
The complexation of Cou-BP-TP and zinc chloride was investigated by UV-vis spectra. As seen in Fig. 2, with the addition of varying amount of zinc chloride to a solution of Cou-BP-TP (5.0×10-5mol/L), the absorptionpeak of Cou-BP-TPat 236nm and 330nm gradually enhanced. The former belonged to π → π* transition while the latter belonged to n → π* transition, which confirmed the interaction between Cou-BP-TP and Zn2+.
|
Download:
|
| Fig. 2. UV-vis spectra of complex Cou-BP-TP + ZnCl2 | |
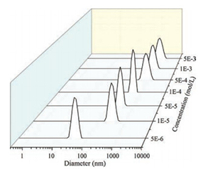
Based on the above results, it can be summarized that the formation of main chain supramolecular polymer p-Cou-BP-TP was due to the interaction between Cou unit and γ-CD as well as the TP unit and ZnCl2. In addition, we performed DLS and TEM experiments of Cou-BP-TP/ZnCl2 system to research the nanoparticle size and morphology of p-Cou-BP-TP. As seen in Fig. 3, the DLS data of p-Cou-BP-TP system decreased significantly with the increasing concentration of polymer. At the concentration of 1.0 × 10-3mol/L, the DLS data reachedto1000nm, which indicated the formation of main-chain supramolecular polymer p-Cou-BPTP.
|
Download:
|
| Fig. 3. DH values of different concentrations of polymers (calculated from the repeat units) obtained by DLS, 25 ℃. | |
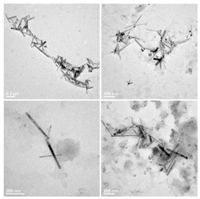
From the following transmission electron microscopy (TEM) images (Fig. 4), we can confirm the existence of supramolecular polymer with the length of 300-400nm on average. It is clear that the appropriate amount of γ-CD and Zn2+ facilitate the existence of linear supramolecular polymer.
|
Download:
|
| Fig. 4. TEM image of the linear polymer p-Cou-BP-TP (2.0×10-5mol/L) | |
In summary, we have successfully constructed a novel linear main-chain supramolecular polymer based on the interaction between Cou unit and γ-CD as well as TP unit and Zn2+ in aqueous solution, and investigated its self-assembly behavior and morphological property. This new strategy to combine host-guest inclusion and metal-ligand coordination has theoretical significance and potential application value, which can improve the development of supramolecular polymer via multiple noncovalent interactions.
AcknowledgmentsWe gratefully acknowledge the financial support from the National Natural Science Foundation of China (NSFC, Nos. 21421004, 21722603 & 21476075), the Innovation Program of Shanghai Municipal Education Commission and the Fundamental Research Funds for the Central Universities (No. 222201717003).
Appendix A. Supplementary dataSupplementary data associatedwith this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.11.045.
| [1] |
C. Fouquey, J.M. Lehn, A.M. Levelut, Adv. Mater. 2 (1990) 254-257. DOI:10.1002/(ISSN)1521-4095 |
| [2] |
P. Wei, X. Yan, F. Huang, Chem. Soc. Rev. 44 (2015) 815-832. DOI:10.1039/C4CS00327F |
| [3] |
J. Zhan, Q. Li, Q. Hu, et al., Chem. Commun. 50 (2014) 722-724. DOI:10.1039/C3CC47468B |
| [4] |
Y. Liu, Y. Yu, J. Gao, Z. Wang, X. Zhang, Angew. Chem. Int. Ed. 122 (2010) 6726-6729. DOI:10.1002/ange.201002415 |
| [5] |
F. Wang, X. Li, S. Wang, et al., Chin. Chem. Lett. 27 (2016) 1592-1596. DOI:10.1016/j.cclet.2016.04.020 |
| [6] |
J.F. Xu, Y.Z. Chen, D. Wu, et al., Angew. Chem. Int. Ed. 52 (2013) 9738-9742. DOI:10.1002/anie.201303496 |
| [7] |
N.N. Adarsh, P. Dastidar, Chem. Soc. Rev. 41 (2012) 3039-3060. DOI:10.1039/c2cs15251g |
| [8] |
Z. Wu, N. Song, R. Menz, et al., Nanomedicine 10 (2015) 1493-1514. DOI:10.2217/nnm.15.1 |
| [9] |
S. Burattini, B.W. Greenland, W. Hayes, et al., Chem. Mater. 23 (2011) 6-8. DOI:10.1021/cm102963k |
| [10] |
D.W. Zhang, X. Zhao, Z.T. Li, Acc. Chem. Res. 47 (2014) 1961-1970. DOI:10.1021/ar5000242 |
| [11] |
X.Y. Hu, T. Xiao, C. Lin, F. Huang, L. Wang, Acc. Chem. Res. 47 (2014) 2041-2051. DOI:10.1021/ar5000709 |
| [12] |
X. Ma, H. Tian, Acc. Chem. Res. 47 (2014) 1971-1981. DOI:10.1021/ar500033n |
| [13] |
T.T. Cao, X.Y. Yao, J. Zhang, Q.C. Wang, X. Ma, Chin. Chem. Lett. 26 (2015) 867-871. DOI:10.1016/j.cclet.2015.01.032 |
| [14] |
F.S. Han, M. Higuchi, D.G. Kurth, Adv. Mater. 19 (2007) 3928-3931. DOI:10.1002/(ISSN)1521-4095 |
| [15] |
C. Xu, Y. Chen, H.Y. Zhang, Y. Liu, J. Photochem. Photobiol. A 331 (2016) 240-246. DOI:10.1016/j.jphotochem.2015.04.014 |
| [16] |
J.P. Byrne, J.A. Kitchen, T. Gunnlaugsson, Chem. Soc. Rev. 43 (2014) 5302-5325. DOI:10.1039/C4CS00120F |
| [17] |
Q. Zhang, D.H. Qu, J. Wu, et al., Langmuir 29 (2013) 5345-5350. DOI:10.1021/la4012444 |
| [18] |
N.A. Tavenor, M.J. Murnin, W.S. Horne, J. Am. Chem. Soc. 139 (2017) 2212-2215. DOI:10.1021/jacs.7b00651 |
| [19] |
P.C. Knipe, S. Thompson, A.D. Hamilton, Chem. Sci. 6 (2015) 1630-1639. DOI:10.1039/C4SC03525A |
 2018, Vol. 29
2018, Vol. 29 


