b Guangdong Tianan New Material Co., Ltd., Foshan 528000, China
Graphene oxide (GO) has become the current global research focus since most reported promising graphene-based advanced materials should be originated from GO due to the difficulty and the high cost for acquiring stable graphene nanosheets as the raw material [1-4]. For example, graphene hydrogels/aerogels (GHs/ GAs) have been discovered to possess outstanding chemical and physical performance [5-7], and show intriguing application prospect in the areas such as catalysts [8], absorbents [9], and supercapacitors [10]. The current effective synthesis route for GHs/ GAs is to make GO nanosheets self-assemble in a hydrothermal reduction system at a high temperature [6, 11] or through a chemical crosslinking method [12, 13]. Obviously, it is deserved to make clear the compositional or morphological changes in GO nanosheets in various reaction environments in order to control the physicochemical behavior and create novel graphene-based advanced materials.
γ-Ray is a kind of ionizing radiation, which has been widely used for the production of a wide range of functional materials from inorganic nanoparticles [14, 15] to macroscopic polymers [16, 17] by taking advantage of the diverse radiation chemical effect of matters. Recently, some researchers reported that GO nanosheets dispersed in dimethyl formamide or the aqueous solution of isopropanol and ethylene diamine can be reduced under γ-ray radiation [18-20], and even can self-assemble to form GH and GA [21]. Although the reduction of GO has been considered mainly to be attributed to the strong reductive hydrated electron and the scavenging effect of isopropanol or amine on oxidative radical (HO·) generated by the water radiolysis, the reduction and selfassembly mechanisms of GO nanosheets in aqueous systems under γ-ray radiation are still unclear since different results from these systems are often reported by researchers [18, 22]. t-Butanol is generally accepted as an effective scavenger for HO· and, to a lesser extent, H· [23]. However, the reduction of GO nanosheets in t-butanol/water medium is inconspicuous, unlike the common behavior of GO nanosheets in other aliphatic alcohol/water media [18, 24].
Herein, in this work, the reduction and self-assembly behavior of GO nanosheets under γ-ray radiation in t-butanol/water were studied in detail in order to reveal a general self-assembly mechanism of GO nanosheets in alcohol-water media under γ-ray radiation and explore new facile and economical preparation method for functionalized GH and GA. First, GO nanosheets were prepared by a modified Hummer's method [25] (Part Ⅰ in Supporting information). The thickness of GO nanosheets is about 0.9 nm measured from the atomic force microscope (AFM) observation (Fig. 1a), similar to the data in other reported works (0.8–1.2 nm) [26, 27]. They can be dispersed uniformly in t-butanol/ water solution with a wide range of pH (2-11), forming a stable brown dispersion. Then, the dispersion of GO was irradiated by 60Co γ-ray after being deoxygenated by N2 (part Ⅱ in Supporting information). The change in the appearance of the t-butanol/water dispersion of GO nanosheets under γ-ray radiation is shown in Fig. 1b. After being irradiated by γ-ray at an absorbed dose of 334.1 kGy, the appearance of the t-butanol/water dispersion of GO with a pH lower than 2 is totally different from that with a higher pH value. A self-standing GH is formed in the strong acidic medium (pH 2), while little change can be observed in those medium with a pH higher than 2.

|
Download:
|
| Fig. 1. (a) The appearance of t-butanol/water dispersion of GO, AFM image and height distribution of GO nanosheets. (b) The appearance of t-butanol/water dispersions of GO with pH 2 and 11 respectively after γ-ray radiation (dose rate: 87 Gy/min, total absorbed dose: 334.1 kGy) and the FTIR spectra of GO nanosheets before and after irradiation. | |
Fig. 1b also displays the FTIR spectra of GO nanosheets before and after γ-ray radiation. The pristine GO exhibits the strong absorption peaks representing the stretching vibration of O-H and carbonyl (C=O) at 3417.5 and 1728.1 cm-1, respectively. The peak at 1623.6 cm-1 is assigned to the absorbed water molecules. The peaks at 1401.2, 1220.6, and 1055.4 cm-1 correspond to the deformation of O-H, the stretching vibration of C-O (epoxy) and C-O (alkoxy), respectively [28]. After γ-ray radiation, the absorption peak related to carbonyl groups (1728.1 cm-1) almost disappears on the FTIR spectra of the irradiated products from both strong acidic and basic t-butanol/water solution, indicating an efficient reduction of C=O induced by γ-ray radiation. It is worth noting that new peaks between 2970–2850 cm-1 assigned to the characteristic stretching vibrations of C-H, as well as the feature peaks belonging to the C-H bending vibration of -CH3 groups at 1381.1 and 1368.5 cm-1, are also clearly observed on the radiated products from both strong acidic and basic media, demonstrating that aliphatic chains have been introduced on the GO nanosheets. The above results indicate that GO nanosheets dispersed in either acidic or basic t-butanol/water solution will go through similar chemistry reaction process under γ-ray radiation, but exhibit the pH-dependent assembly behavior, which results in totally different product morphologies.
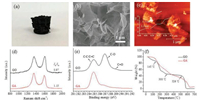
Significantly, a self-standing macroporous graphene aerogel (GA) with a low density of about 4 mg/cm3 can be obtained after the freeze-drying of the GH produced from the t-butanol/water solution with pH 2, as shown in Figs. 2a and b. The AFM image of the nanosheets in GA is shown in Fig. 2c. It can be seen that a basic thickness of the nanosheets forming GA is about 1.2–1.3 nm, which is a little bit higher than that of the pristine GO nanosheets (0.9 nm). The chemical structure of the GA is further analyzed by Raman and X-ray photoelectron spectroscopy (XPS) spectra, as shown in Figs. 2d and e, respectively. Commonly, the ID/IG ratio on the Raman spectrum of GO can be used for characterizing the reduction of GO [29]. The ID/IG ratio of the prepared GA is 1.15, higher than that of the pristine GO (0.97), suggesting that GO nanosheets have been reduced to a certain extent after γ-ray radiation [18]. The change of the atomic ratio and the combinative status of C and O elements on GO nanosheets detected by XPS can also reflect the reduction of GO [30]. It is seen in Fig. 2e that the intensities of C1s peaks at 286.8 eV (C-O) and 288.4 eV (C=O) on pristine GO nanosheets all decrease dramatically after γ-ray radiation. The C/O ratio also increases greatly from 1.8 of the pristine GO to 5.6 of GA. The thermal gravimetric analysis (TGA) curve of GA measured in air is shown in Fig. 2f, compared with that of the pristine GO. Evidently, the weight loss process of GA is quite different from that of GO. A remarkable weight loss of the oxygencontaining groups on GO appears before 300 ℃, while GA exhibits a slow and continuous weight loss after 150 ℃, indicating the loss of a large part of oxygen-containing groups on the nanosheets after γ-ray radiation. The above results imply that the prepared GA is mainly constituted of reduced GO (rGO) nanosheets.
|
Download:
|
| Fig. 2. The digital photo (a), scanning electron microscope (SEM) image (b), AFM image (c), Raman spectrum (d), XPS spectrum (e), and TGA curves (f) of GA prepared by freeze-drying the GH produced in t-butanol/water (pH 2) under g-ray radiation (dose rate: 87 Gy/min, total absorbed dose: 334.1 kGy). | |
Based on the above results, the self-assembly mechanism of GO nanosheets in the strong acidic t-butanol/water solution should be necessarily discussed since it is significant to develop a new preparation method of functional graphene-based aerogel.
It is well-known that the main primary active species produced from the γ-ray radiolysis of pure water in an inert atmosphere include H·, HO·, and eaq- according to the classic radiation chemistry theory [23, 31]. In the strong acidic t-butanol/water solution, the primary radiolysis active species will experience the following reactions:

|
(1) |
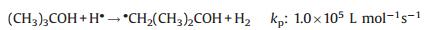
|
(2) |
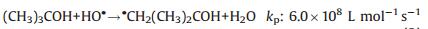
|
(3) |
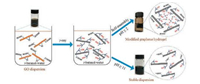
It means all the primary active species will rapidly transform into alcohol radicals (·CH2(CH3)2COH), which have enough chemical reactivity to react with the reactive sites of GO nanosheets [18]. As a result, the GO nanosheets will be reduced and hydroxyalkylated simultaneously under γ-ray radiation, as illustrated in Fig. 3. The reduction and hydroxyalkylation effect will make the radiated nanosheets become amphiphilic due to the hydrophilicity of hydroxyl and the hydrophobicity of alkyl and reduced GO nanosheets. At a strong acidic condition, the concentration of ·CH2(CH3)2COH is relatively high since eaq- will totally transform into H·, resulting in a higher hydroxyalkylation degree of GO nanosheets. The abundant -OH groups on the nanosheets can form hydrogen bonds with water molecules. Under the combination interaction of strong π-π conjugation between the reduced GO nanosheets and the formation of hydrogen bonds, the self-assembly of the radiation-modified nanosheets is triggered, and a free-standing monolithic GH is formed. On the other hand, at a strong basic condition, the hydroxyaklylation degree of GO nanosheets is relatively low since the concentration of primary radiolysis active free radicals which can be transformed into ·CH2(CH3)2COH is lower than that at a strong acidic condition. At the same time, the stability of the irradiated GO nanosheets will be improved due to their negative zeta potential caused by the ionization of the remained -COOH groups. Thus, only a stable modified GO nanosheets dispersion can be obtained after γ-ray radiation on a strong basic t-butanol/water dispersion of GO.
|
Download:
|
| Fig. 3. The formation of reduced and hydroxyalkylated GO nanosheets in t-butanol/water solution under γ-ray radiation and their different behavior at strong acidic (pH ≤ 2) and basic (pH ≥ 11) conditions. | |
It should be noted that previous work show that GA can be prepared from the aqueous solution of other water-soluble aliphatic alcohols, such as ethanol, isopropanol, etc., through the direct freeze-drying or self-assembly process under γ-ray radiation [21, 32]. But there is no report on the preparation of GA from t-butanol/water medium till now. Based on the above proposed mechanism, the reaction of alcohol radicals and GO nanosheets is the key process for the formation of the self-standing GH. It is known that the reaction constants of (CH3)3COH with HO· and H· are 1 ~2 orders of magnitude lower than those of other alcohols, and the steric hindrance of ·CH2(CH3)2COH is greater than other aliphatic alcohol radicals [33]. As a result, a large absorbed dose higher than 250 kGy is needed for an enough hydroxyalylation degree to trigger the self-assembly of the nanosheets, as shown in Fig. 4. Therefore, it confirms that GO nanosheets will be hydroxyalkylated due to the attack of the alkyl alcohol radicals in all aliphatic alcohol/water solution. The degree of hydroxyalkylation determines the pH-dependent aggregation behavior of GO nanosheets.

|
Download:
|
| Fig. 4. The photos of the t-butanol/water dispersion of GO (pH 2) and the products after being irradiated by γ-ray radiation at different absorbed dose. | |
In this work, the chemical structure change and self-assembly behavior of GO nanosheets in t-butanol/water medium under γ-ray radiation were investigated. The FTIR, Raman, and XPS spectra of the radiated GO nanosheets show that GO nanosheets will be reduced and hydroxyalkylated simultaneously by the alcohol free radicals produced by the reaction of t-butanol and the primary active species of water radiolysis. The hydroxyalkylated GO nanosheets will be triggered to self-assemble into a stable hydrogel at a low pH value (≤2) driven by the strong π-π conjugation between the reduced GO nanosheets and the hydrogen bonds between -OH groups on GO nanosheets and water molecules. After the freeze-drying of hydrogel, a free-standing graphene aerogel can be obtained. This work provides a general and facile method for the preparation of hydroxylalkylated graphene-based aerogel.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 51573175, 51473152, 51773189), Foshan Scientific and Technological Innovation Team Project (No. 2013- IT100041), Foshan University-City Cooperation Project (Scientific and Technological Innovation Project, No. 2014HK100291), and the Fundamental Research Funds for the Central Universities (Nos. WK2060200012, WK3450000001).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.11.003.
| [1] |
D.R. Dreyer, S.J. Park, C.W. Bielawski, R.S. Ruoff, Chem. Soc. Rev. 39 (2010) 228-240. DOI:10.1039/B917103G |
| [2] |
S.F. Pei, H.M. Cheng, Carbon 50 (2012) 3210-3228. DOI:10.1016/j.carbon.2011.11.010 |
| [3] |
D. Chen, H.B. Feng, J.H. Li, Chem. Rev. 112 (2012) 6027-6053. DOI:10.1021/cr300115g |
| [4] |
D. Voiry, J. Yang, J. Kupferberg, et al., Science 353 (2016) 1413-1416. DOI:10.1126/science.aah3398 |
| [5] |
M.A. Worsley, P.J. Pauzauskie, T.Y. Olson, et al., J. Am. Chem. Soc. 132 (2010) 14067-14069. DOI:10.1021/ja1072299 |
| [6] |
Y.X. Xu, K.X. Sheng, C. Li, G.Q. Shi, ACS Nano 4 (2010) 4324-4330. DOI:10.1021/nn101187z |
| [7] |
Z.F. Zhao, X.B. Wang, J.L. Qiu, et al., Rev. Adv. Mater. Sci. 36 (2014) 137-151. |
| [8] |
J. Li, C.Y. Liu, Y. Liu, J. Mater. Chem. 22 (2012) 8426-8430. DOI:10.1039/c2jm16386a |
| [9] |
H.Y. Sun, Z. Xu, C. Gao, Adv. Mater. 25 (2013) 2554-2560. DOI:10.1002/adma.201204576 |
| [10] |
Y.X. Xu, Z.Y. Lin, X.Q. Huang, et al., Adv. Mater. 25 (2013) 5779-5784. DOI:10.1002/adma.v25.40 |
| [11] |
K.W. Hu, X.Y. Xie, T. Szkopek, M. Cerruti, Chem. Mater. 28 (2016) 1756-1768. DOI:10.1021/acs.chemmater.5b04713 |
| [12] |
H. Bai, C. Li, X.L. Wang, G.Q. Shi, J. Phys. Chem. C 115 (2011) 5545-5551. |
| [13] |
H. Hu, Z.B. Zhao, Z.B. Wan, Y. Gogotsi, J.S. Qiu, Adv. Mater. 25 (2013) 2219-2223. DOI:10.1002/adma.201204530 |
| [14] |
P. Chen, L.Y. Song, Y.K. Liu, Y. Fang, Radiat. Phys. Chem. 76 (2007) 1165-1168. DOI:10.1016/j.radphyschem.2006.11.012 |
| [15] |
A. Abedini, A.R. Daud, M.A.A. Hamid, N.K. Othman, PLoS ONE 9 (2014) e90055. DOI:10.1371/journal.pone.0090055 |
| [16] |
S. Baccaro, C. Casieri, A. Cemmi, et al., Radiat. Phys. Chem. 141 (2017) 131-137. DOI:10.1016/j.radphyschem.2017.06.017 |
| [17] |
M.M. Ghobashy, M.A. Elhady, Radiat. Phys. Chem. 134 (2017) 47-55. DOI:10.1016/j.radphyschem.2017.01.021 |
| [18] |
B.W. Zhang, L.F. Li, Z.Q. Wang, et al., J. Mater. Chem. 22 (2012) 7775-7781. DOI:10.1039/c2jm16722k |
| [19] |
Y.W. Zhang, H.L. Ma, Q.L. Zhang, et al., J. Mater. Chem. 22 (2012) 13064-13069. DOI:10.1039/c2jm32231e |
| [20] |
J.H. Li, B.W. Zhang, L.F. Li, et al., Radiat. Phys. Chem. 94 (2014) 80-83. DOI:10.1016/j.radphyschem.2013.06.029 |
| [21] |
Y.L. He, J.H. Li, L.F. Li, J.Y. Li, Mater. Lett. 177 (2016) 76-79. DOI:10.1016/j.matlet.2016.04.187 |
| [22] |
H.L. Ma, L. Zhang, Y.W. Zhang, et al., Acta Phys.-Chim. Sin. 31 (2015) 2016-2022. |
| [23] |
G.V. Buxton, C.L. Greenstock, W.P. Helman, A.B. Ross, J. Phys. Chem. Ref. Data 17 (1988) 513-886. DOI:10.1063/1.555805 |
| [24] |
L. Shahriary, A. Athawale, Bull. Mater. Sci. 38 (2015) 739-745. DOI:10.1007/s12034-015-0889-9 |
| [25] |
W.S. Hummers, R.E. Offeman, J. Am. Chem. Soc. 80 (1958) 1339-1339. DOI:10.1021/ja01539a017 |
| [26] |
S. Stankovich, D.A. Dikin, R.D. Piner, et al., Carbon 45 (2007) 1558-1565. DOI:10.1016/j.carbon.2007.02.034 |
| [27] |
S.S. Nanda, G.C. Papaefthymiou, D.K. Yi, Crit. Rev. Solid State Mater. Sci. 40 (2015) 291-315. DOI:10.1080/10408436.2014.1002604 |
| [28] |
D.N. He, Z. Peng, W. Gong, et al., RSC Adv. 5 (2015) 11966-11972. DOI:10.1039/C4RA14511A |
| [29] |
L.Q. Xie, Y.H. Zhang, F. Gao, et al., Chin. Chem. Lett. 28 (2017) 41-48. DOI:10.1016/j.cclet.2016.05.015 |
| [30] |
Y.L. Guo, H.C. Jin, Z.Z. Du, X.W. Ge, H.X. Ji, Chin. J. Chem. Phys. 30 (2017) 461-466. DOI:10.1063/1674-0068/30/cjcp1703062 |
| [31] |
C.J. Hochanadel, J. Phys. Chem. 56 (1952) 587-594. DOI:10.1021/j150497a008 |
| [32] |
W.K. Wang, Y.H. Wu, Z.W. Jiang, et al., Appl. Surf. Sci. 427 (2018) 1144-1151. DOI:10.1016/j.apsusc.2017.09.058 |
| [33] |
H. W. Richter, Radiation chemistry: principles and applications, in: J. F. Wishart, D. G. Nocera (Eds. ), Photochemistry and Radiation Chemistry, American Chemical Society, Akron, 1998, pp. 5-33.
|
 2018, Vol. 29
2018, Vol. 29 


