In the past decades, various stimulus-responsive polymeric nanostructures (e.g., micelles, vesicles, nanogel) and hydrogels are receiving great attention in biomedical applications [1-3]. Owing to excellent biocompatibility, biodegradability, and easy-to-access modification and functionalization, synthetic polypeptides and their copolymers have been widely used for engineering stimulusresponsive soft materials [4]. These polypeptide-based biomaterials hold great potentials in drug, protein, DNA or siRNA delivery systems, anti-microbial treatments, and tissue engineering scaffolds [4-8].
In the case of thermosensitive polypeptide, Li et al. systematically investigated the thermosensitive phase behavior and the conformation transition of poly(methoxydiethylene glycol-L-glutamate) and other poly(L-glutamate)s with long methoxyethylene glycol segment [9, 10]. Chen et al. synthesized the thermosensitive oligo(ethylene glycol)-derived poly(L-glutamate) by using a click grafting method [11]. We found that star-shaped poly(methoxydiethylene glycol-L-glutamate) without other hydrophobic segments or terminal groups could form thermosensitive hydrogel via the micellar aggregation mechanism [12]. As for the photosensitive polypeptides and copolymers, the spiropyrancontaining block copolymer poly(L-glutamic acid)-b-poly(ethylene oxide) (PEO) can induce a reversible aqueous micellization dissolution process due to the phototriggered spiropyran-tomerocyanine isomerization upon UV (350 nm) or visible light (590 nm) irradiation [13]. Zhao et al. studied the 6-bromo-7- hydroxycoumarin-4-ylmethyl-modified poly(L-glutamic acid)-b-PEO copolymer, which presented an interesting near infrared light triggered micellar disruption in aqueous solution [14]. We reported that poly(S-(o-nitrobenzyl)-L-cysteine)-b-PEO could be used for the fabrication of photo-redox-sensitive nano-vesiclesfor stimulitriggered intracellular drug release [15, 16].
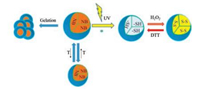
Very recently, Lu and Li et al. reported a novel class of polypeptide bearing azobenzene and oligoethylene glycol of different lengths, which exhibits irreversible aqueous thermosensitivity and reversible UV–vis-induced conformation and organogel-sol transitions [17]. However, the studies on multi-stimuliresponsive polypeptide nanostructure and hydrogel are scarce, which are expected to provide a platform to study the structurefunction correlation of copolypeptide and hold promising for various biomedical applications. We reason that the random copolypeptide composed of S-(o-nitrobenzyl)-L-cysteine (NBC) and methoxydiethylene glycol-L-glutamate (EG2-Glu) units intrinsically have photo-thermo-sensitivity and the decagedthiol group on L-cysteineunit simultaneously enables it have redox-sensitivity [15, 16]. In this work, we synthesize a series of random copolypeptides by ring-opening copolymerization of NBC-Ncarboxyanhydride (NBC-NCA) and EG2-Glu-NCA in dried DMF. The random copolypeptides present quadruple thermo-photoredox-responsive aqueous self-assembly properties and form a supramolecular hydrogel (Scheme 1), holding potentials for drug delivery and injectable hydrogel for soft tissue engineering.
|
Download:
|
| Scheme 1. Quadruple stimuli-responsive aqueous self-assembly and supramolecular gelation of one random copolypeptide. | |
A series of random copolypeptidesof poly(methoxydiethylene glycol-L-glutamate)-co-poly(S-(o-nitrobenzyl)-L-cysteine) with different compositions was synthesized by ring-opening copolymerization of both NBC-NCA (N) and EG2-Glu-NCA (E), in which the copolypeptide was denoted as ExNy (both x and y in subscript represents the degree of polymerization) in supporting information (Table S1 in Supporting information). 1H NMR spectroscopy was used to determine the composition of the resulting copolypeptide and the degree of polymerization (Fig. S1 in Supporting information). The typical proton signals at 7.4–8.0 ppm are characteristic o-nitrobenzyl group of NB, that at 3.3–3.4 ppm belongs to the methoxy group of pendant methoxydiethylene glycolgroup (CH3O-), and those at 4.3–4.4 ppm (a) and 4.5–4.6 ppm (a') respectively represents the methinebackbone (CH) of L-glutamate unit and L-cysteine unit. Based on the integral ratio of a/a', the monomer compositionwithin copolypeptideis basically consistent with that in feed ratio. The GPC traces of the copolypeptides verified that the pure product with monodisperse distribution was successfully prepared (Fig. S2 in Supporting information). Note that the copolymer with increasing L-cysteine composition shifted to a relatively lower molecular weight region due to the enhanced β-sheet conformation stabilized by intermolecular interactions [15].
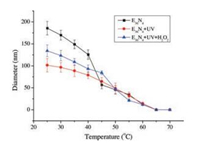
The random copolypeptides consist of hydrophobic photoresponsive NBC units and hydrophilic thermosensitive EG2-Glu units, and the NB-caged thiol group on L-cysteine unit has latent redox-sensitivity [15, 16]. Hence, the random copolypeptides are expected to self-assemble into multi-stimuli-responsive nanoparticles in aqueous solution. As we know, the lower critical solution temperature (LCST) of the diethylene glycol-derived poly (L-glutamate) systems is dependent on secondary structure, the length of side chain and polymer concentration [9, 10]. Herein, the effect of copolypeptide composition (i.e., hydrophilicity-hydrophobicity) on LCST of copolypeptide is studied by a turbidity method and a temperature-dependent dynamic light scattering (DLS). The temperature point at 90% light transmittance is used to denote LCST (Table S1). In general, the increasing hydrophobic composition in copolymer leads to a smaller LCST and a quicker phase transition in aqueous solution. With the NBC composition in random copolypeptide increased from 10 mol% to 50 mol%, the LCST dropped from 47.8 ℃ to 35.0 ℃. Taking E36N4 (10 mol% NBC monomer) as an example (Fig. 1), the temperature-dependent DLS results demonstrated that the self-assembled copolypeptide nanoparticles shrank over the increasing temperature, which was similar to the amphiphilic homopolypeptide poly(methoxydiethylene glycol-L-glutamate) counter part [12]. The nanoparticles would finally aggregate and precipitate from aqueous solution above 60 ℃. In all, the copolypeptide nanoparticles present an aqueous thermosensitive behavior similar to the homopolypeptide counterpart.
|
Download:
|
| Fig. 1. The temperature-dependent diameters of the assembled nanoparticles including E36N4, the UV-irradiated ones, and the UV + H2O2 stimulated ones. | |
The photosensitive behavior of the copolypeptide nanoparticle in aqueous solution was tested under the irradiation of 365 nm UV-lamp. Taking E36N4 as example, the maximal broad absorbance peak at about 310–350 nm gradually increased over the irradiation time and then nearly unchanged after 15 min and the nanoparticles solution grew slightly yellow (Fig. S3 in Supporting information). These findings indicate that the NB group was increasingly photocleaved into nitrosobenzylaldehyde until full cleavage, demonstrating that the copolypeptide nanoparticles have a fast UV-photosensitivity in aqueous solution [15]. The photosensitivity of the copolypeptide nanoparticle was further verified by on-line DLS and TEM (Fig. 2). Before exposure to UV irradiation, the copolypeptide E36N4 self-assembled into nearly globular nanoparticle with an average DLS diameter of about 185 nm while the nanoparticle reduced to 112 nm after 15 min of UV irradiation. The hydrophobic nitrobenzyl groups within the aggregated polypeptide core were fully cleaved, which induced the size reduction of the nanoparticles. Especially, the on-line DLS results showed that the nanoparticle quickly reassembled to small ones within 3 min irradiation and then the reassembled nanoparticles dynamically balanced from 3 min to 15 min. This was because 85 mol% of hydrophobic NB groups in nanoparticles were photocleaved in 3 min, which played a decisive role for the reassembly process due to the hydrophobicity-hydrophilicity balance associated with the NB cleavage [15, 16]. The TEM data further confirmed the selfassembled copolypeptide nanoparticles were reduced to a small size after 15 min UV irradiation.

|
Download:
|
| Fig. 2. The DLS diameter of the E36N4 nanoparticle dependent on irradiation time (A), the TEM images of nanoparticle before (B) and after 15 min UV irradiation (C). | |
It is known that the oxidation of thiol and the disulfide bond exchange reaction can be used to construct reversible cross-linked nanostructures including nanogels [18]. Thus, the photocleaved copolypeptide E36N4 nanoparticles containing multiple thiol pendant L-cysteine unitsin aqueous solution can be further transformed into the disulfide-bond cross-linked nanogels. When a little amount of H2O2 (0.1 mmol/L) is added to the nanoparticle solution, the nanoparticles containing multiple thiol groups underwent a disulfide-bond-forming process to form the crosslinked nanogels, during which the nanoparticles size slightly increasedtoabout 134 nm after 16 h (Fig. 3). This was because some inter-particles cross-linking to some extent occurred, which resulted in some larger nanogels [16]. Note that the thiol groups are mainly embedded in the nanogels, the possible cross-linking between nanogels might be negligible. The disulfide-bond crosslinks can be reversibly broken into free thiols by DTT reduction (10 mmol/L), the nanogel was accordinglyreduced to small ones of about 110 nm at 16 h. These results indicate that the redox reaction mainly occurred in intra-nanoparticles, during which the nanogel size slightly changed in aqueous solution. The photosensitivity was mainly induced by the cleavage reaction, so the photosensitive nanoparticle is non-cycled. However, the thiol-disulfide bond exchange reaction is dynamically reversible and the oxidized or reduced nanoparticles can be reversibly converted.

|
Download:
|
| Fig. 3. The diameter of the photocleaved E36N4 nanoparticles dependent on the H2O2 oxidation time (A) and the DTT reduction time (B) in aqueous solution. | |
As for E36N4 sample, the semitransparent supramolecular hydrogel formed at a concentration of ~2.9 wt% while the suspension solution was obtained for the copolypeptides with high hydrophobic NBC compositions (e.g., E30N10 and E32N8). As we know, both G' and G" represent storage and loss modulus of the hydrogel, respectively. The G' of the E36N4 hydrogel was about an order of magnitude higher than the G", exhibiting a typical elastomeric behavior (Fig. 4A). Moreover, the G0 increased over frequency within 40 rad/s, which was probably due to a weak and heterogeneous network existed in the hydrogel [12]. Finally the G' kept nearly constant at about 80 Pa within the measured frequency of 100 rad/s. Note that the random copolypeptide E36N4 was terminated by a benzyl amine initiator. Similarly, the copolypeptide p-E36N4 terminated with a small propargyl group was prepared, which formed a relatively stronger and homogeneous hydrogel with a G' of about 250 Pa (Fig. 4B). These rheological analyses indicated the terminal group has an apparent effect on the mechanical property of the supramolecular copolypeptide hydrogel, which was probably due to the hydrophilicity-hydrophobicity balance in aqueous solution [19]. In contrast, the homopolypeptide of poly(methoxydiethylene glycol-L-glutamate) is very difficult to form a soft hydrogel or needs a very higher concentration [12], which demonstrates that the hydrophobic NBC units of random copolypeptide also play an important role for forming the hydrogel network. In addition, a higher hydrophobic NBC composition would induce aqueous macro-phase separation for the random copolypeptides such as E30N10 and E32N8 samples. Note that the detailed mechanism of both the terminal group and the hydrophobic composition effects on forming copolypeptide hydrogel deserves to be further investigated.

|
Download:
|
| Fig. 4. Frequency-sweep rheological measurements for the supramolecular hydrogels of E36N4 (A) and p-E36N4 (B). | |
We have successfully synthesized a series of random copolypeptides of poly(methoxy-diethylene glycol-L-glutamate)-co-poly (S-(o-nitrobenzyl)-L-cysteine) with different compositions, which demonstrates quadruple stimuli-responsive aqueous self-assembly behavior. Specifically, the self-assembled nanoparticles would shrink over temperature while they dynamically reassembled into small ones upon UV irradiation. The irradiated nanoparticles then formed a disulfide-bond cross-linked nanogel, exhibiting a reversible redox-responsivity upon sequential H2O2 oxidation and DTT reduction. The random copolypeptide finally can form supramolecular hydrogel with weak mechanical modulus and both the hydrophobic unit composition and the terminal group play a decisive role for the hydrogel formation.
AcknowledgmentThe authors are grateful for the financial support of the National Natural Science Foundation of China (No. 21474061).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.09.042.
| [1] |
L.H. Wang, C.Y. Hong, Acta Polym. Sin. 2 (2017) 200-213. |
| [2] |
D. Wang, Y. Jin, X. Zhu, D. Yan, Prog. Polym. Sci. 64 (2017) 114-153. DOI:10.1016/j.progpolymsci.2016.09.005 |
| [3] |
W. Lu, X. Le, J. Zhang, Y. Huang, T. Chen, Chem. Soc. Rev. 46 (2017) 1284-1294. DOI:10.1039/C6CS00754F |
| [4] |
T.J. Deming, Prog. Polym. Sci. 32 (2007) 858-875. DOI:10.1016/j.progpolymsci.2007.05.010 |
| [5] |
C.H. Cai, J.P. Lin, Y.Q. Lu, Q. Zhang, L.Q. Wang, Chem. Soc. Rev. 45 (2016) 5985-6012. DOI:10.1039/C6CS00013D |
| [6] |
C. Deng, J. Wu, R. Cheng, et al., Prog. Polym. Sci. 39 (2014) 330-364. DOI:10.1016/j.progpolymsci.2013.10.008 |
| [7] |
H. Lu, J. Wang, Z. Song, et al., Chem. Commun. 50 (2014) 139-155. DOI:10.1039/C3CC46317F |
| [8] |
H. Tian, Z. Tang, X. Zhuang, X. Chen, X. Jing, Prog. Polym. Sci. 37 (2012) 237-280. DOI:10.1016/j.progpolymsci.2011.06.004 |
| [9] |
Y. Shen, X.H. Fu, W. Fu, Z.B. Li, Chem. Soc. Rev. 44 (2015) 612-622. DOI:10.1039/C4CS00271G |
| [10] |
X.H. Fu, Y.N. Ma, J. Sun, Z.B. Li, Chin. J. Polym. Sci. 34 (2016) 1436-1447. DOI:10.1007/s10118-016-1861-x |
| [11] |
Y. Cheng, C. He, C. Xiao, et al., Polym. Chem. 2 (2011) 2627-2634. DOI:10.1039/c1py00281c |
| [12] |
D.L. Liu, X. Chang, C.M. Dong, Chem. Comm. 49 (2013) 1229-1231. DOI:10.1039/c2cc38343h |
| [13] |
V.K. Kotharangannagari, A.J. Sa'nchez-Ferrer, R. Ruokolainen Mezzenga, Macromolecules 45 (2012) 1982-1990. DOI:10.1021/ma2026379 |
| [14] |
S. Kumar, J.F. Allard, D. Morris, et al., J. Mater. Chem. 22 (2012) 7252-7257. DOI:10.1039/c2jm16380b |
| [15] |
G. Liu, C.M. Dong, Biomacromolecules 13 (2012) 1573-1583. DOI:10.1021/bm300304t |
| [16] |
G. Liu, L. Zhou, Y. Guan, Y. Su, C.M. Dong, Macromol. Rapid. Commun. 35 (2014) 1673-1678. DOI:10.1002/marc.v35.19 |
| [17] |
W. Xiong, X. Fu, Y. Wan, et al., Polym. Chem. 7 (2016) 6375-6382. DOI:10.1039/C6PY01364C |
| [18] |
M. Huo, J.Y. Yuan, L. Tao, Y. Wei, Polym. Chem. 5 (2014) 1519-1528. DOI:10.1039/C3PY01192E |
| [19] |
C. Chen, D. Wu, W. Fu, Z.B. Li, Biomacromolecules 14 (2013) 2494-2498. DOI:10.1021/bm4008259 |
 2018, Vol. 29
2018, Vol. 29 


