b Institute of Electron Devices and Circuits, Ulm University, Ulm 89069, Germany
Boron-doped nanocrystalline diamond (BNCD) shows unique and superior performance in many ways such as biocompatibility, transparency and high chemical as well as electrochemical stability, giving a wide potential window ranging from -2.0 V to +2.0 V, with small background current and corrosion resistance [1-4]. Because of these properties, the BNCD is an attractive material for a wide range of applications. It plays especially an important role in the electrochemical analysis and electrochemical treatment of wastewater [5-7], where the relevant electrochemical analysis includes organic and inorganic detection, biological analysis, trace metal composition detection, and others.
Diamond-based microelectrode arrays (MEAs) [8, 9] have been used as a tool in the field of excitatory cell research for many years because of their small sizes and high sensitivity. Meanwhile, reactive oxygen species (ROS), which comprise oxygen anions (O2-), molecular hydrogen peroxide (H2O2), hydroxyl radicals (OH·), etc. [10], play a crucial role in the generation and development of cancer cells [11-15]. Because H2O2 has a relatively long lifetime, it can be used as an important indicator to elucidate the content of ROS in cells. It is known that a respiratory burst can produce large amounts of H2O2 when cells are stimulated, and then it will start cleaning and efflux mechanisms to restore the normal concentration of H2O2.
Based on these observations, in this study we have developed new boron-doped nanocrystalline diamond microelectrode arrays (BNCD-MEAs) [16] with 16 channels to detect biological signals from some activated cancer cells. Since the concentration of intracellular H2O2 generated from the metabolism of oxygen radical, oxidase enzymes catalytic reaction, and others, usually keeps a value favored for cellular proliferation, artificial stimulation like ascorbic acid (AA) exerted on cells could disrupt intracellular redox homeostasis, which can readily lead to the efflux of H2O2 from target cells. Upon recordings of the released H2O2 from different cells, including normal cells and cancer cells stimulated by AA [17, 18], it can readily detect the ROS released from target cells, which will be helpful for the cancer cell recognition and also beneficial for further studying the cause of relevant disease [19]. In this contribution, we have demonstrated the possibility for the high sensitive electrochemical determination of cellular flux of H2O2 from single cell or a few cells on exposure to AA by using the as-prepared BNCD-MEAs.
In the experiments, all reagents were purchased from Sigma - Aldrich Co. The water used in all experiments is ultrapure water (MilliQ, Millipore Ltd., U.S.A.). In the electrochemical experiments, phosphate buffered saline (PBS) (0.02 mol/L, pH 7.4) was used as electrolyte. In order to eliminate dissolved oxygen in the solution, high-purity nitrogen was bubbled for about 20 min before the measurements. The content of H2O2 was calibrated by a standard method using potassium permanganate solution. All electrochemical experiments (i.e., cyclic voltammetry (CV) and amperometric study (I-t)) were conducted by using a custom-made glass cell consisting of a diamond MEA as working electrode, an Ag/AgCl reference electrode, and a platinum (Pt) wire counter electrode, respectively.
In this work, we designed new BNCD-MEAs, which contain 16 channels (16-Ch). The MEAs were fabricated by a micro technological procedure, which can be briefly described as follows. Firstly, a 25 × 25 mm2 sample of glass AF32eco(Schott, Mainz, Germany) was prepared. The surface was cleaned first by subsequent sonication in acetone and isopropanol and then by oxygen plasma. After that the sample was seeded with a nanodiamond suspension. Then a 1 μm thick insulating nanocrystalline diamond (NCD) film, acting as buffer layer, was grown onto it by chemical vapor deposition (CVD) using methane as precursor gas. After that a 200 nm thick BNCD layer was overgrown under similar conditions. Afterwards, by means of optical lithography and reactive ion etching (RIE), the patterns of 16 chips with 5.5 × 5.5 mm2 were transferred to the BNCD-layer. Sequentially, a ~2.5 μm thick parylene passivation layer was added on top grown by CVD at room temperature. For opening the active BNCD areas of the bonding pads and the 20 mm wide electrodes, again optical lithography and RIE were applied. The diced chips were finally assembled by flipchip with silver epoxy glue EPO-TEK H20E (Polytec PT GmbH, Waldbronn, Germany) onto appropriate carriers and provided with a glass ring, which created an area to culture cells and perfusion chamber of convenient volume.
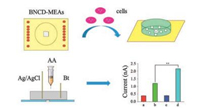
The human liver cancer cells (HepG2) and normal liver cells (L02) are respectively cultured on the as-prepared BNCD-MEAs within the glass ring. During this study, the cells were cultured with the Dulbecco's modified eagle medium (DMEM) containing 10% fetal bovine serum, and put in the 37 ℃ incubator with 5% of CO2 and a relative humidity of 95%. After 8 h incubation, it could be observed that cells were adhered to the surface of the BNCD-MEAs.
When the cells covered all electrodes, the medium was carefully removed, and the BNCD-MEAs were washed with PBS at least 3 times. Before starting the relevant detection, 2 mL PBS was added into the glass ring. Afterwards, AA was added to the solution, and electrochemical measurements were performed under N2 atmosphere, as shown in Fig. 1. It was necessary to maintain N2 flow rate to allow the complete diffusion of solution.
|
Download:
|
| Fig. 1. Cells were cultured in the BNCD-MEAs. Study of MEAs with different cells upon addition of AA. | |
When the BNCD-MEAs have been designed, microscopic structure characterization is essential for understanding the electrode performance. Fig. 2A shows a scanning electron microscope (SEM) image of the as-prepared BNCD-MEAs. The thickness of the BNCD conducting layer is only about 200 nm. Fig. 2B shows the sensing area of the MEAs, with the 16 semitransparent microelectrodes (red circle), plated with L02 cells. Since the effective electrode area is very limited, only single cell can be docked on the electrode. Fig. 2C shows the designed appearance of finalized 16-Ch MEAs. Our studies indicate that it is convenient to culture cells or store the solution in the glass ring and the electrode regeneration is simple. When adding solution in the glass ring, it is desirable to keep the reference and counter electrode in contact with the solution so that we can readily get informative signals without waiting for long equilibration transients. Our studies demonstrate that the relevant standard cyclic voltammetry (CV) curves of 16 microelectrodes are similar, and the stability, reproducibility and repeatability of the boron-doped nanocrystalline diamond microelectrode arrays are excellent.

|
Download:
|
| Fig. 2. (A) Typical SEM image of diamond electrode materials in Cross section. (B) Sensing area of the semi-transparent 16Ch-MEAs plated with L02 cells. The diameter of effective electrode marked by the red circle is 20 μm. Scale bar: 60 μm. (C) Finalized 16-Ch MEAs. Two sides are foreseen, left: the glass ring, with 16 contact pads, and right: connections for all 16 electrodes. | |
Based on these observations, the specificity or selectivity of the as-prepared MEAs has been further explored. With regards to the interferences from the related chemicals, we have performed relevant studies pertaining to H2O2, AA, GSH, and others, under identical experimental conditions, and found that only H2O2 could lead to the changes of peak current. So we can use the relevant I-t response of BNCD-MEAs in target cells to demonstrate the performance of ROS release from the stimulated cells (Fig. S1 in Supporting information).
As we know, normal cells and cancer cells are different in some specific characteristics, such as different ROS levels (including O2-, H2O2, OH-, etc.) in the relevant cells. Therefore, we can use the MEAs to measure the amount of H2O2 released from different cells stimulated by AA. Fig. 3A shows the recorded amperometric I-t curve for the current response in PBS at 0 V upon addition of AA to stimulate the H2O2 from HepG2 cells. The as-prepared MEAs has 16 channels so that it provides 16 I-t curves of the relevant measurements from the target HepG2 cancer cells. All electrodes have different changes in the related I-t curves, but they have the same variation tendency. The HepG2 cancer cells release H2O2 after adding AA to stimulate cells. When more amount of AA was added more H2O2 released from the cells was observed, just like Fig. 3A demonstrates, the first arrow indicates a point where 10 μL of 1 mmol/L AA is added, while the second one clarifies the point where 10 μL of 2 mmol/L AA is added. Because the amount of the cells on the surface of the MEAs is limited, i.e., single cell or a few cells around per electrode, the amount of H2O2 released from the stimulated cells is also limited. Meanwhile, different kinds of cells also released different amount of H2O2 when stimulated by AA, and HepG2 cancer cells release much more H2O2 than that of L02 normal cells under the identical experimental conditions (Fig. 3B). The considerable difference of relevant currents indicates that the related cancer cells can release much more H2O2 than that of normal cells, which could be further adapted to distinguish cancer cells from normal cells (Fig. S2 in Supporting information).

|
Download:
|
| Fig. 3. Study of MEA with different cells upon addition of AA at 0 V in N2-saturated PBS (0.1 mol/L, pH 7.4). (A) AA was added twice to 2 mL PBS with HepG2 cells, and the concentration of AA was 1 mmol/L and 2 mmol/L, successively. (B) The current values of different cells: a. L02 cells, b. L02 cells with adding AA, c. HepG2 cells, d. HepG2 cells with adding AA. ** means significantly differences (n = 5 each, P < 0.01, t-test). | |
In summary, in this study we have fabricated the boron-doped nanocrystalline diamond MEAs with 16-Ch and exploited the possibility of the simultaneous bio-analysis for the rapid recognition of cancer cells. It is evident that accompanying with the apparent advantages of MEAs, a variety of data can be obtained and thus rule out the contingency of experiments resulted from the different electrodes. The results demonstrate that the as-prepared diamond microelectrode arrays can be readily utilized to detect H2O2 released from normal cells and cancer cells like HepG2 cells when stimulated with AA. The considerable difference of relevant currents indicates that the related cancer cells can release much more H2O2 than that of normal cells, which could be further adapted to distinguish cancer cells from normal cells. It is evident that the as-prepared diamond MEAs can be not only conveniently used in the rapid cancer cell detection but also be utilized for other relevant bio-analysis applications. Due to the appropriate size of the 16-Ch electrode arrays (20 μm, i.e., the common size of mammal somatic cells), in our next research, we can use the microarrays to investigate the oxidative stress behaviors of single cell.
AcknowledgmentsThis work was financially supported by the National High Technology Research and Development Program of China (No. 2015AA020502), the National Natural Science Foundation of China (Nos. 81325011, 21175020, 21327902), the Fundamental Research Funds for the Central Universities of China (No. 2242016K41023), and the Project "Strategic Partnerships U5" of Ulm University, Ulm, Germany, which is funded by the German Academic Exchange Service (DAAD) and the German Ministry for Education and Research (BMBF).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.10.037.
| [1] |
P.Y. Lim, F.Y. Lin, H.C. Shih, V.G. Ralchenko, Thin Solid Films 516 (2008) 6125-6132. DOI:10.1016/j.tsf.2007.11.016 |
| [2] |
Y.V. Pleskov, Prot. Met. 42 (2006) 103-118. DOI:10.1134/S0033173206020019 |
| [3] |
R. Ramesham, M.F. Rose, Corros. Sci. 39 (1997) 2019-2033. DOI:10.1016/S0010-938X(97)00093-0 |
| [4] |
M.B. Brahim, H.B. Ammar, R. Abdelhédi, Y. Samet, Chin. Chem. Lett. 27 (2016) 666-672. DOI:10.1016/j.cclet.2015.12.032 |
| [5] |
R. Fardel, U. Griesbach, H. Putter, C. Comninelli, J. Appl. Electrochem. 36 (2006) 249-253. DOI:10.1007/s10800-005-9057-z |
| [6] |
P.A. Michaud, M. Panizza, L. Ouattara, T. Diaco, J. Appl. Electrochem. 33 (2003) 151-154. DOI:10.1023/A:1024084924058 |
| [7] |
A.M. Polcaro, A. Vacca, S. Palmas, M. Masciaaa, J. Appl. Electrochem. 33 (2003) 885-892. DOI:10.1023/A:1025815828503 |
| [8] |
W. Li, S.W. Xu, H.R. Xu, et al., Chin. Chem. Lett. 27 (2016) 738-744. DOI:10.1016/j.cclet.2016.01.018 |
| [9] |
V. Carabelli, A. Marcantoni, F. Picollo, A. Battiato, E. Bernardi, ACS Chem. Neurosci. 8 (2017) 252-264. DOI:10.1021/acschemneuro.6b00328 |
| [10] |
S. Borgmann, Anal. Bioanal. Chem. 394 (2009) 95-105. DOI:10.1007/s00216-009-2692-1 |
| [11] |
Z. Yan, W.C. Yu, Z.X. Jiao, et al., Nanoscale 5 (2013) 1816-1819. DOI:10.1039/c3nr33954h |
| [12] |
H.C. Chang, X.M. Wang, K.K. Shiu, et al., Biosens. Bioelectron. 41 (2013) 789-794. DOI:10.1016/j.bios.2012.10.001 |
| [13] |
A.L. Sanford, S.W. Morton, K.L. Whitehouse, et al., Anal. Chem. 82 (2010) 5205-5210. DOI:10.1021/ac100536s |
| [14] |
C.J. Gao, H.M. Zhu, J. Chen, H.D. Qiu, Chin. Chem. Lett. 28 (2017) 1006-1012. DOI:10.1016/j.cclet.2017.02.011 |
| [15] |
Y.Y. Zhang, C.Y. Wu, H. Jiang, J.L. Zuo, X.M. Wang, Sci. Chi. Chem. 58 (2015) 1193-1199. DOI:10.1007/s11426-015-5352-7 |
| [16] |
T.C. Granado, G. Neusser, C. Kranz, A. Pasquarelli, Phys. Status Solidi A 212 (2015) 2445-2453. DOI:10.1002/pssa.201532168 |
| [17] |
L.Y. Ping, L.H. Qing, R. Qi, T. Yang, Anal. Chem. 81 (2009) 3035-3041. DOI:10.1021/ac802721x |
| [18] |
P. Wu, Z. Cai, J. Chen, C. Cai, Biosens. Bioelectron. 26 (2011) 4012-4017. DOI:10.1016/j.bios.2011.03.018 |
| [19] |
J.Y. Li, Y.X. Shi, S. Matthias, et al., Sci. Chi. Chem. 57 (2014) 833-841. DOI:10.1007/s11426-014-5092-0 |
 2018, Vol. 29
2018, Vol. 29 


