b State Key Laboratory of the Discovery and Development of Novel Pesticide, Shenyang Sinochem Agrochemicals R & D Co., Ltd., Shenyang 110021, China
Rice sheath blight (Rhizoctonia solani Kühn, RSB) is one of the three important diseases (rice sheath blight, bacterial blight and rice blast) of rice. Meanwhile, sorghum anthracnose (Colletotrichum graminicola (Cesati) Wilson, SA) is also a worldwide disease, which occurs at all stages of sorghum growth. It was reported that both RSB and SA can cause 10%–30% even 40%–50% production loss in crops per year worldwide [1, 2]. The use of fungicides can effectively reduce crop loss and further to guarantee crop harvest. At present, the existing pesticides for control of RSB are mainly tebuconazole, pyraclostrobin and azoxystrobin; and thiram and prochloraz for control of SA. However, the fungus will develop resistance subsequently after these agrochemicals are used for a while. The Fungicide Resistance Action Committee (FRAC) reported that the fungicides with known modes of action are classified according to target sites into more than 50 groups [3]. Unfortunately over 80% target sites have already developed medium to high resistance risk to many familiar classes of fungicides, which involved the vast majority of fungicide structures. So it is an urgent demand for developing continually novel and highly active fungicides with different modes of action to address the increasingly serious resistance problem.
1, 3, 4-Oxadiazole derivatives have been paid more and more attention since Gibson reported cyclization mechanism of this type of compounds in 1962 firstly [4]. The derivatives of 1, 3, 4-oxadiazoles often exhibit broad biological activities in medicine [5-9] and agriculture. For agrochemicals, Song et al. [10], introduced phenoxymethyl moiety into 1, 3, 4-oxadiazole to afford insecticides; Zhang et al. [11], discovered antimicrobial agents by importing phenylpyrazolyl to 1, 3, 4-oxadiazole. Zhang et al. [12], inserted piperazine to 1, 3, 4-oxadiazole and obtained compounds which exhibited broad spectrum herbicidal and fungicidal activities. Some literatures also disclosed 1, 3, 4-oxadiazoles containing phenyl [13], pyridyl [14], thiazole ring [14] as agrochemicals. On the other side, the arylpyrazole derivatives have been playing an important role both in medicinal [15, 16] and crop protection fields [17], too. The main varieties containing arylpyrazole substructure include pyrametostrobin [18, 19], pyraoxystrobin [20] and pyraclostrobin [21, 22]. Although these three fungicides all belong to strobilurin, they focus on different targets, rice sheath blight and rice blast, downy mildew, and powdery mildew, respectively. Scholars have been carrying out studies on taking arylpyrazole as structural units of pesticides. Wang et al. [23], found pyridylpyrazole acid derivatives can be used as lead compounds for further development of novel insecticides. Wu et al. [24], demonstrated that arylpyrazole carboxamide derivatives exhibited broad-spectrum insecticidal activities. Wang et al. [25], achieved heterocyclic compounds containing pyridylpyrazole not only displayed excellent fungicidal activities, but also exhibited modest insecticidal activities. Therefore, arylpyrazole is a magical and formidable chemical group, which is worthy of being applied further.
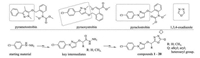
In this study, to develop new fungicides with high biological activity, based on the excellent performance of the above two kinds of compounds, under the direction of the intermediate derivatization methods (IDM) [26-32], we introduced arylpyrazoloxyl to 1, 3, 4-oxadiazole and designed a series of novel 1, 3, 4-oxadiazole derivatives using 4-chlorophenylhydrazine as the starting material (Scheme 1). The detailed synthesis, bioassays and structureactivity relationship of these derivatives were discussed below.
|
Download:
|
| Scheme 1. An overview of the design of 1, 3, 4-oxadiazole derivatives. | |
All chemicals such as starting materials and reagents were commercially available (Sinopharm Chemical Reagent Co., Ltd., China) and used without further purification. Melting points were determined on a Büchi M-569 melting point apparatus and were uncorrected. 1H NMR and 13C NMR spectra were recorded with a Mercury 300 MHz spectrometer with deuterochloroform (CDCl3) or DMSO-d6 as the solvent and tetramethylsilane (TMS) as the internal standard. Elemental analyses were determined on an elementar Vario EL cube instrument. The mass spectra were acquired with an Agilent 1100 series LC/MSD Trap equipped with electron spray ionization (ESI) source. X-ray structure determination was recorded with Bruker D8 Quest Single crystal X-ray diffractometer. The isolation of the compounds was conducted by Biotage Isolera Prime flash purification system.
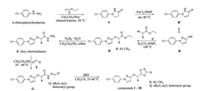
The general synthetic methods for compounds 1–20 are shown in Scheme 2. Intermediates A and B/B' were prepared according to the previously reported methods [33]. The general procedures for synthesis of intermediates D, E, G and compounds 1–20 were listed in Supporting information.
|
Download:
|
| Scheme 2. Synthetic route of compounds 1–20. | |
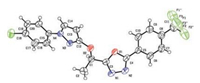
The crystal structure of target compound 4 was also determined by X-ray diffraction analyses (The atomic coordinates for compound 4 have been deposited at the Cambridge Crystallographic Data Centre. CCDC ID: 1572037 contain the supplementary crystallographic data for this article. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.). Its crystal structure is shown in Fig. 1.
|
Download:
|
| Fig. 1. X-ray single-crystal diffraction of compound 4. | |
The antifungal activities in vitro of all the synthesized compounds against two plant pathogenic fungi (RSB and SA) were conducted by the mycelium linear growth rate method as previously reported [34]. The final concentration of the test compound in the culture medium was 100, 50, 25, 12.5, 6.25 and 3.125 mg/L. For details, see Supporting information. The test results of the fungicidal activities of compounds 1 to 20 against RSB and SA are listed in Table 1.
|
|
Table 1 Chemical structures and fungicidal activity of 1, 3, 4-oxadiazole derivatives (compounds 1–20). |
According to the scheme described in Scheme 2, twenty title compounds were designed and synthesized, their chemical structures were summarized in Table 1. The synthesized compounds were characterized by 1H NMR, 13C NMR, MS and elemental analyses. The chemical structure of compound 4 was also unequivocally determined by X-ray crystallography (Fig. 1).
In vitro bioassays indicated that these compounds have moderate to significant fungicidal activity against RSB and SA. Especially, compounds 4, 16 and 20 displayed excellent activities against RSB (EC50 = 0.88, 0.91 and 0.85 mg/L, respectively), higher than the reference tebuconazole; While compound 3 exhibited higher activity against SA (EC50 = 1.03 mg/L), equal to commercial pyraclostrobin (EC50 = 1.02 mg/L). In general, the fungicidal activity of the tested compounds against RSB is superior to that against SA, so the following structure–activity relationships (SAR) were mainly unfolded around RSB.
Initially, in order to verify the feasibility of our design concept, compounds 1–3 were designed and synthesized employing simple raw materials available in our lab. Fortunately, they indicated certain fungicidal activity with EC50 values of 25.87 mg/L, 2.94 mg/L and 1.57 mg/L, respectively. The results suggested that phenyl, particularly the p-substituted phenyl, maybe play an important role in the interaction of active molecules with targets. Encouraged by this finding, further structural modifications around p-position on phenyl were carried out. The typical electron-withdrawing groups (CF3 and NO2, compounds 4 and 6 respectively) were designed. The bioassay results showed that the EC50 values were 0.88 mg/L and 2.16 mg/L respectively, especially compound 4 showed higher activity than the reference tebuconazole (EC50 = 0.88 mg/L versus 1.02 mg/L). However, the introduction of the NO2 (compound 6), a stronger electron-withdrawing group led to a decrease of the activity (EC50 = 2.16 mg/L). On the contrary, the replacement of electron-donating group methoxyl (compound 7, EC50 = 1.97 mg/L), bulky moiety t-butyl (compound 9, EC50 = 1.92 mg/L) and greater steric group 2, 4, 6-(trimethyl) (compound 11, EC50 = 12.46 mg/L) did not make any contribution on bioactivity compared with compound 4.
Next, to determine if changing other representative substituents to replace Q would bring enhanced fungicidal activity, we synthesized compounds 12 (Q = cyclopropyl), 14 (Q = n-propyl), 16 (Q = phenylethyl), 17 (Q = 2-thiophenyl), 19 (Q = 2-pyridinyl) and 20 (Q = 3-pyridinyl). To our surprise, compounds 16 and 20 displayed prominent control effect with EC50 of 0.91 mg/L and 0.85 mg/L, respectively, nearlyclose to commercial pyraclostrobin (0.81 mg/L), a little higher than tebuconazole, which implied that compounds bearing Q of phenylethyl and 3-pyridinyl are worthy of being novel leads for further study as well. As for R substituent, by comparing the relevant compound pairs, we found the activity of compounds possessingRofHagainstRSB isusuallybetterthanthatofcompounds imparting R of methyl except compound pair of 4 and 5 showing comparative EC50 values (0.88 mg/L and 0.89 mg/L, respectively).
In summary, twenty novel 1, 3, 4-oxadiazole derivatives were designed and synthesized. In vitro bioassays showed that these compounds have moderate to significant fungicidal activity against RSB and SA. Three compounds (4, 16 and 20) were discovered preliminarily after hit–to–lead optimization with EC50 values of 0.85–0.91 mg/L against RSB, comparable to tebuconazole (1.02 mg/L). Compound 3 displayed higher activity against SA (EC50 = 1.03 mg/L), which is also equal to commercial pyraclostrobin (EC50 = 1.06 mg/L). This study demonstrated that 1, 3, 4-oxadiazole derivatives can be further studied as lead compounds for control of rice sheath blight and/or sorghum anthracnose. Compound 20 is a promising fungicide for further development. Further syntheses and structural optimization studies are in progress.
AcknowledgmentThis work was financially supported partially by the Open Foundation of Key Laboratory for Anisotropy and Texture of Materials, Ministry of Education, Northeastern University, China (No. ATM20170001).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.01.050.
| [1] |
L. Meng, Chemical Control of Rice Sheath Blight and Effect Evaluation of Several Pharmaceutical Compounds (Dissertation), Nanjing Agricultural University, Nanjing, 2013 p. 1.
|
| [2] |
L.P. Teng, L. Yu, J. Gao, J. Henan Agric. Sci. 41 (2012) 80-83. |
| [3] |
FRAC (Fungicide Resistance Action Committee) Code List 2017, Fungicides Sorted by Mode of Action (Including FRAC Code Numbering); www. frac. info (Accessed December 01, 2017).
|
| [4] |
M.S. Gibson, Tetrahedron 18 (1962) 1377-1380. DOI:10.1016/S0040-4020(01)99292-0 |
| [5] |
A.U. Rehman, S. Ahtzaz, M.A. Abbasi, et al., J. Chil. Chem. Soc. 62 (2017) 3370-3375. DOI:10.4067/S0717-97072017000100013 |
| [6] |
D. Szulczyk, P. Tomaszewski, M. Jozwiak, et al., Molecules 22 (2017) 49. |
| [7] |
D. Kerzare, R. Chikhale, R. Bansode, et al., J. Braz. Chem. Soc. 27 (2016) 1998-2010. |
| [8] |
M.U. Islam, M. Albratty, J. Chem. Bio. Phys. Sci. Sec. A 6 (2016) 624-633. |
| [9] |
M.S.Y. Khan, G. Chawla, M.A. Mueed, Indian J. Chem. Sec. B 43 (2004) 1302-1305. |
| [10] |
B.A. Song, J.X. Chen, D.Y. Hu, et al., CN Patent (2017), 106432125. |
| [11] |
T.T. Zhang, P.Y. Wang, J. Zhou, et al., J. Heterocycl. Chem. 54 (2017) 2319-2325. DOI:10.1002/jhet.2820 |
| [12] |
Y. Zhang, X.H. Liu, Y.Z. Zhan, et al., Bioorg. Med. Chem. Lett. 26 (2016) 4661-4665. DOI:10.1016/j.bmcl.2016.08.059 |
| [13] |
X.M. Zheng, Z. Li, Y.L. Wang, et al., J. Fluorine Chem. 123 (2003) 163-169. DOI:10.1016/S0022-1139(03)00168-4 |
| [14] |
J.C. Liu, W.D. Wang, H.W. He, Chin. J. Org. Chem. 34 (2014) 1447-1451. DOI:10.6023/cjoc201403013 |
| [15] |
R. Sven, P. Josef, S. Thorsten, et al., WO Patent (2011), 2011092187. |
| [16] |
J.F. Lu., CN Patent (2012), 102558058. |
| [17] |
X.F. Sun, J.F. Tian, M.N. Zhu, B.S. Chai, C.L. Liu, Chin. J. Pestic. 50 (2011) 781-789. |
| [18] |
X.F. Cao, J.L. Liu, Z.N. Li, M.S. Ji, Chin. J. Pestic. 49 (2010) 323-325343. |
| [19] |
C. MacBean, The pesticide manual, 16th ed., BCPC, Alton, UK, 2012 p. 966.
|
| [20] |
C. MacBean, The pesticide manual, 16th ed., BCPC, Alton, UK, 2012 p. 967.
|
| [21] |
C. MacBean, The pesticide manual, 16th ed., BCPC, Alton, UK, 2012pp. 963-964.
|
| [22] |
P. Mcdougall, AgriService/AgriReference/Products (Pyraclostrobin), Phillips McDougall AgriService, 2015 http://www.phillipsmcdougall.co.uk/. (accessed Dec. 02, 2017) p. 220.
|
| [23] |
B.L. Wang, H.W. Zhu, Y. Ma, et al., J. Agric. Food Chem. 61 (2013) 5483-5493. DOI:10.1021/jf4012467 |
| [24] |
Z.B. Wu, X. Zhou, Y.Q. Ye, P.Y. Wang, S. Yang, Chin. Chem. Lett. 28 (2017) 121-125. DOI:10.1016/j.cclet.2016.06.010 |
| [25] |
B.L. Wang, H.W. Zhu, Z.M. Li, et al., Pest Manage. Sci. 74 (2018) 726-736. DOI:10.1002/ps.2018.74.issue-3 |
| [26] |
C.L. Liu, Chin. J. Pestic. 50 (2011) 20-22. |
| [27] |
C.L. Liu, A.Y. Guan, J.D. Yang, et al., J. Agric. Food Chem. 64 (2016) 45-51. DOI:10.1021/jf5054707 |
| [28] |
A.Y. Guan, C.L. Liu, X.P. Yang, M. Dekeyser, Chem. Rev. 114 (2014) 7079-7107. DOI:10.1021/cr4005605 |
| [29] |
A.Y. Guan, C.L. Liu, G. Huang, et al., J. Agric. Food Chem. 61 (2013) 11929-11936. DOI:10.1021/jf403739e |
| [30] |
A.Y. Guan, C.L. Liu, M. Li, et al., Pest Manage. Sci. 67 (2011) 647-655. DOI:10.1002/ps.v67.6 |
| [31] |
H.C. Li, A.Y. Guan, G. Huang, et al., Bioorg. Med. Chem. 24 (2016) 453-461. DOI:10.1016/j.bmc.2015.09.032 |
| [32] |
A.Y. Guan, C.L. Liu, W. Chen, et al., J. Agric. Food Chem. 65 (2017) 1272-1280. DOI:10.1021/acs.jafc.6b05580 |
| [33] |
H. König, N. Götz, U. Klein, K. Eller, US Patent 5922886, 1999.
|
| [34] |
R. Yang, Z.F. Gao, J.Y. Zhao, et al., J. Agric. Food Chem. 63 (2015) 1906-1914. DOI:10.1021/jf505609z |
 2018, Vol. 29
2018, Vol. 29