b Tianjin International Joint Academy of Biotechnology and Medicine, Tianjin 300457, China;
c Patent Examination Cooperation Jiangsu Center of the Patent Office, SIPO, Suzhou 215163, China
The invention of antibiotics was regarded as one of the greatest medicinal improvements in the 20th century, because thousands of patients had been rescued from fatal bacterial infection. However, with the abuse of antibiotics, resistance against these drugs to bacteria has become a global problem gradually. In 2008, Rice termd "the ESKAPE bugs" to describe Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumonia, Acinetobacter baumanni, Pseudomonas aeruginosa and Enterobacter as the most common resistant bacteria species in hospitals [1]. In 2010, a carbapenemase named New Delhi metallo-beta-lactamase-1 (NDM-1), was reported to cause Gram-negative bacterial resistance to almost all available β-lactam antibiotics and the rapid spread of the plasmid-encoded NDM-1 gene in some countries subsequently indicated that "superbug" era was arriving [2, 3].
NDM-1 belongs to the class B metallo-β-lactamase (MBL) superfamily, which contains enzymes that hydrolyze β-lactams and cleaves the β-lactam ring of the compound, leading to antibiotic-resistant infections [4]. Crystal structures of NDM-1 had been elucidated by different research groups, either the free enzyme alone or that in a bound form [4-8]. Two catalytic zinc ions have been found in the active site of the enzyme, which play essential roles for the biochemical function of this metallolactamase. The NDM-1 crystal structures were bound either with the hydrolyzed state of an antibiotic drug, or an inhibitor such as Lcaptopril. These achievements have not only provided structural insights into β-lactam recognition and inhibition mechanism, but also offered opportunities to design novel inhibitors targeting NDM-1 in a rational way [7, 8].
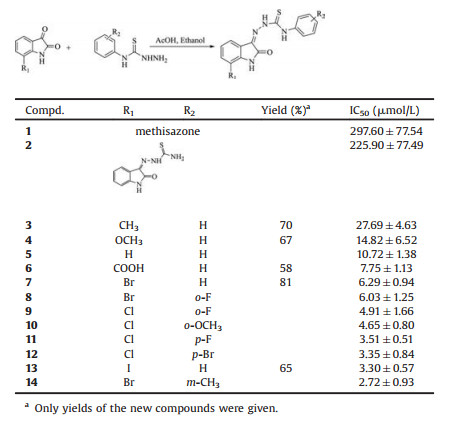
Very few medicinal antibiotics can inhibit resistant bacteria with NDM-1 gene effectively, including colistin and tigecycline. From 2000 to 2013 only 22 new antibiotics were approved, but none of them was indicated for Gram-negative bacteria [9]. It is therefore a strong demand to develop novel inhibitors tackle such a bacterial resistance. Rao et al. reported that captopril is an NDM-1 inhibitor with IC50 of 7.9 μmol/L [4], based on which some captopril analogues were synthesized and evaluated [10]. Proschak et al. accessed some approved drugs containing thiols and identified some good NDM-1 inhibitors [6]. Aspergillomarasmine A (AMA) was shown to be a rapid and potent NDM-1 inhibitor, the IC50 of which is 4.0 μmol/L [11]. Ebselen was reported to be a potent covalent inhibitor of NDM-1, which binds to Cys211 to inactivate the enzyme [12]. Cheng et al. discovered some active compounds via multistep virtual screening and the most potent inhibitor VNI-41 has an IC50 of 29.6 μmol/L [13]. Yang et al. demonstrated that triazolylthioacetamide is a promising scaffold for the development of NDM-1inhibitors, and the best IC50 value is 0.15 μmol/L for compound 4a in their study [14].
Isatin is an endogenous natural product and its derivatives possess a wide range of biological activities, such as antibacterial, antitubercular, antimalarial, antifungal and antiviral activities [15-20]. Some drugs contain isatin as an active scaffold, for instance, indirubin and methisazone, which have been used to treat chronic myelogenous leukemia and smallpox infection, respectively [21, 22]. It is a common knowledge that the most fruitful basis for the discovery of a new drug is to start with an old drug [6, 23]. In the course of screening new chemical agents for resistant bacteria, we had evaluated the inhibitory activity of some medicines containing isatin structure against purified NDM-1. Methisazone was found to display some activity in the screening process, although the IC50 value for NDM-1 of this old drug is only 297.6 μmol/L. Inspired by this result, we then synthesized some isatin-β-thiosemicarbazone (IBT) derivatives designed from methisazone and tested their in vitro inhibitory activities. Enhanced biological potencies were observed for the IBT compounds and many IC50 values against NDM-1 are at micromolar level. Thus we have identified for the first time that a series of IBT compounds are new NDM-1 inhibitors, which is meaningful for further drug discovery. Fig. 1 illustrated the chemical structures of some reported NDM-1 inhibitors, isatin, indirubin and methisazone.
|
Download:
|
| Fig. 1. Chemical structures of reported NDM-1 inhibitors and some drugs containing isatin scaffold. | |
The synthetic method for the IBT compounds was similar to our previous procedure, in which the target compounds were chemically prepared by a straightforward condensation of isatin and thiosemicarbazide [24]. The yields of the reaction were generally satisfactory, in the range of 65% ~ 80%. For examples, the yield of compound 6 is 58% while that of compound 7 is 81%. The synthetic scheme, chemical structures of compounds 1-14 and their in vitro inhibitory activities against NDM-1 are shown in Table 1. The inhibitory potencies of the IBT derivatives are sorted in ascending order. Compounds 3, 4, 6, 7 and 13 are new compounds that have not been characterized before, whereas the other compounds had been reported and detailed elsewhere [24-26]. Detailed experimental procedures, analytical data for all previously unreported compounds, 1H NMR, 13C NMR and HRMS figures for selected compounds, relevant biological data and inhibition curves for all NDM-1 inhibitors are in Supporting information.
|
|
Table 1 IBT NDM-1 inhibitors and their biological activities. |
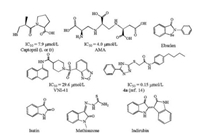
From the enzymatic inhibitory data, it can be seen that methisazone (compound 1) exhibits a weak IC50 of 297.60 μmol/L. Compound 2 has a close structure to methisazone and the IC50 data improves to 225.9 μmol/L. When a phenyl ring is attached to the thiourea part of the compound, there is a significant improvement of the biological activity. Compounds 3-14 display desirable NDM-1 inhibition data, among which compound 14 is the strongest one, with an IC50 value of 2.72 μmol/L. Compound 3 has an IC50 data of 27.69 μmol/L, compound 4 has an IC50 data of 14.82 μmol/L and the IC50 data for compound 5 is 10.72 μmol/L, respectively. The IC50 data of the other nine compounds (6-14) were < 10 μmol/L. Fig. 2 shows the inhibition curve of compound 14.
|
Download:
|
| Fig. 2. Inhibition curve of compound 14 against NDM-1. | |
The results are interesting because this is the first identification of IBTstructures as NDM-1 inhibitors, indicating their potential use to treat bacterial infections associated with New Delhi metallo-β-lactamase-1, ideally when used in combination with clinical antibiotics. Indeed, some IBT compounds had been recognized as strong inhibitors of Methicillin-resistant Staphylococcus aureus (MRSA) in our previous work, many of which were much better than the control drug vancomycin [24]. Bearing this in mind, soon after we discovered that IBTs are NDM-1 inhibitors, all the fifty-one IBTs in that paper were subjected to NDM-1 assay, however only a few compounds (5, 8–12, 14) were active against NDM-1. Not every IBT derivative displayed both MRSA inhibition and NDM-1 inhibition, therefore there should be no direct relationship between the two different biological activities. The bacterial strains for MRSA are Gram-positive, while NDM-1 only exists in Gram-negative bacteria strains.
Compared with the reported NDM-1 inhibitors, the IBT compounds are distinct in their chemical structures. The IC50 data of most known NDM-1 inhibitors are at micromolar level, similar to the potency of the IBT inhibitors in this study. This means that the IBT compounds studied here are a new family of NDM-1 inhibitors. Methisazone has been used as an old antiviral drug for treating smallpox infection for a half century [22], it is likely that suitable modifications on the structure of methisazone might bring a new drug with clinical safety.
To further understand the structure-activity relationship of these inhibitors, a comparative field analysis (CoMFA) model was constructed subsequently. The leave-one-out q2 is 0.644 at the optimum components of 2, and the non-cross-validated r2 is 0.917, with a standard error of estimate of 0.204. The steric and electrostatic contributions are 59.4% and 40.6%, respectively. Considering the fact that only fourteen IBT compounds are in the database, the statistical result is fairly satisfactory. Compound 14 has been selected as a template to depict the structural features of the NDM-1 inhibitors. For the steric contour map (Fig. 3a), a bulky group will be favorable for better NDM-1 inhibition in the green contour region and such a group is likely to decrease the biological activity in the yellow contour space. The green map is hidden behind the yellow contour region in the phenyl part (in the right side of the figure). For the electrostatic contour map (Fig. 3b), a positively charged group will increase the activity of the compound in the blue contour region, meanwhile, a negatively charged group is favorable to enhance the activity in the red region. It seems that the electrostatic contour map is better than the steric contour map for further molecular design from this CoMFA model. Details for molecular simulation and experimental biological activity versus predicted biological activity from CoMFA model are in Supporting information.

|
Download:
|
| Fig. 3. Three-dimensional contour maps from the CoMFA model of the NDM-1 inhibitors. | |
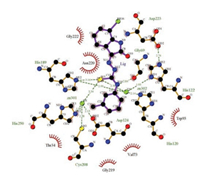
The possible binding mode of the NDM-1 inhibitors of IBT family was then generated via molecular docking simulation. In this procedure 4EXS from protein databank was used because this pdb file contains a reported NDM-1 inhibitor L-captopril [8]. Fig. 4 illustrates the interaction of compound 14 and the neighboring residues of NDM-1 drawn by LIGPLOT [27]. It could be seen that many residues (Thr34, Val73, Trp93, Gly219, Asn220 and Gly222) form hydrophobic interactions with the inhibitor, while two residues (Gly69 and His189) have hydrogen bonding contacts with the inhibitor. Two zinc ions in the structure form several coordination bonds with the sulfur atom in the IBT molecule, suggesting their essential roles in the inhibition process. This is in agreement with the binding modes of some reported NDM-1 inhibitors or hydrolyzed antibiotics, in which the zinc ions also serve as chelating anchors for the biochemical function of this enzyme.
|
Download:
|
| Fig. 4. 2D representation of 14 bound with NDM-1 from molecular docking. | |
In summary, by screening the activity groups on the isatin fragments, isatin-β-thiosemicarbazone scaffold has been studied as novel inhibitors of New Delhi metallo-β-lactamase-1, the best IC50 value was found as low as 2.72 μmol/L. This new class of compounds is different from the published NDM-1 inhibitors, which is interesting for further research. The quantitative structure-activity relationship analysis and the proposed binding mode suggested by molecular docking have provided important clues on further structural modification. In face of the superbug attack, more and more research should be carried out in this area. The present study has hence shed light on the design and discovery of novel NDM-1 inhibitors based on the IBT scaffold.
AcknowledgmentsThis work was supported by the "111" Project of Ministry of Education of China (No. B06005), the National Natural Science Foundation of China (No. 21672114) and the National Basic Research Program of China (No. 2013CB734004).
| [1] |
L.B. Rice, J. Infect. Dis. 197 (2008) 1079-1081. DOI:10.1086/588758 |
| [2] |
K.K. Kumarasamy, M.A. Toleman, T.R. Walsh, et al., Lancet Infect. Dis. 10 (2010) 597-602. DOI:10.1016/S1473-3099(10)70143-2 |
| [3] |
D. Yong, M.A. Toleman, C.G. Giske, et al., Antimicrob. Agents Chemother. 53 (2009) 5046-5054. DOI:10.1128/AAC.00774-09 |
| [4] |
Y. Guo, J. Wang, G. Niu, et al., Protein Cell 2 (2011) 384-394. DOI:10.1007/s13238-011-1055-9 |
| [5] |
Y. Kim, C. Tesar, J. Mire, et al., PLoS One 6 (2011) e24621. DOI:10.1371/journal.pone.0024621 |
| [6] |
F.M. Klingler, T.A. Wichelhaus, D. Frank, et al., J. Med. Chem. 58 (2015) 3626-3630. DOI:10.1021/jm501844d |
| [7] |
D.T. King, L.J. Worrall, R. Gruninger, N.C. Strynadka, J. Am. Chem. Soc. 134 (2012) 11362-11365. DOI:10.1021/ja303579d |
| [8] |
H. Feng, J. Ding, D. Zhu, et al., J. Am. Chem. Soc. 136 (2014) 14694-14697. DOI:10.1021/ja508388e |
| [9] |
F.M. Klingler, D. Moser, D. Büttner, et al., Bioorg. Med. Chem. Lett. 25 (2015) 5243-5246. DOI:10.1016/j.bmcl.2015.09.056 |
| [10] |
N. Li, Y. Xu, Q. Xia, et al., Bioorg. Med. Chem. Lett. 24 (2014) 386-389. DOI:10.1016/j.bmcl.2013.10.068 |
| [11] |
A.M. King, S.A. Reid-Yu, W. Wang, et al., Nature 510 (2014) 503-506. DOI:10.1038/nature13445 |
| [12] |
J. Chiou, S. Wan, K.F. Chan, et al., Chem. Commun. 51 (2015) 9543-9546. DOI:10.1039/C5CC02594J |
| [13] |
X. Wang, M. Lu, Y. Shi, Y. Ou, X. Cheng, PLoS One 10 (2015) e0118290. DOI:10.1371/journal.pone.0118290 |
| [14] |
L. Zhai, Y.L. Zhang, J.S. Kang, et al., ACS Med. Chem. Lett. 7 (2016) 413-417. DOI:10.1021/acsmedchemlett.5b00495 |
| [15] |
Z. Xu, S. Zhang, C. Gao, et al., Chin. Chem. Lett. 28 (2017) 159-167. |
| [16] |
K.H.M.E. Tehrani, M. Hashemi, M. Hassan, F. Kobarfard, S. Mohebbi, Chin. Chem. Lett. 27 (2016) 221-225. DOI:10.1016/j.cclet.2015.10.027 |
| [17] |
T.N. Akhaja, J.P. Raval, Chin. Chem. Lett. 23 (2012) 785-788. DOI:10.1016/j.cclet.2012.05.004 |
| [18] |
T.N. Akhaja, J.P. Raval, Chin. Chem. Lett. 23 (2012) 446-449. DOI:10.1016/j.cclet.2012.01.040 |
| [19] |
H.M. Zhang, H. Dai, P.J. Hanson, et al., ACS Chem. Biol. 9 (2014) 1015-1024. DOI:10.1021/cb400775z |
| [20] |
S.N. Pandeya, S. Smitha, M. Jyoti, S.K. Sridhar, Acta Pharm. 55 (2005) 27-46. |
| [21] |
Z. Xiao, Y. Hao, B. Liu, L. Qian, Leuk. Lymphoma 43 (2002) 1763-1768. DOI:10.1080/1042819021000006295 |
| [22] |
D.J. Bauer, Antimicrob. Agents Chemother. 5 (1965) 544-547. |
| [23] |
X.Y. Ai, H.J. Liu, C. Lu, et al., Theranostics 7 (2017) 425-435. DOI:10.7150/thno.17073 |
| [24] |
X.M. Zhang, H. Guo, Z.S. Li, et al., Eur. J. Med. Chem. 101 (2015) 419-430. DOI:10.1016/j.ejmech.2015.06.047 |
| [25] |
M.D. Hall, N.K. Salam, J.L. Hellawell, et al., J. Med. Chem. 52 (2009) 3191-3204. DOI:10.1021/jm800861c |
| [26] |
D.J. Bauer, P.W. Sadler, Br. J. Pharmacol. Chemother. 15 (1960) 101-110. DOI:10.1111/(ISSN)1476-5381a |
| [27] |
A.C. Wallace, R.A. Laskowski, J.M. Thornton, Protein Eng. 8 (1995) 127-134. DOI:10.1093/protein/8.2.127 |
 2018, Vol. 29
2018, Vol. 29