b Center for Physiochemical Analysis and Measurement, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China;
c University of Chinese Academy of Sciences, Beijing 100049, China
Inspirited by the microstructure of nacre, the materialdesign and large-scale integration of artificial nanofluidic devices step into a completely new stage, termed "2D nanofluidics" [1, 2]. Via the exfoliation-reconstruction strategy, a lamellar configuration can be constructed by restacking the dispersed 2D nanosheets in liquid phase [3, 4]. The interstitial space can be generally considered as lamellar nanochannels that allows the transport of molecular cargoes and ionic species [5]. The nacre-inspired 2D layered membrane provides a solution for large-scale integration of cascading lamellar nanofluidic channels into macroscopic membrane materials for practical use, such as molecular separation, water treatment, and energy storage [6-8]. Besides the most well-studied graphene-based materials [9, 10], other types of 2D nanomaterials [11-15], such as the transition metal dichalcogenides (TMDCs) [16-20], are also expected as promising nano-building-blocks in the fabrication of new 2D nanofluidic devices and materials.
TMDCs are semiconductors of the type MX2, where M is a transition metal atom (such as Mo or W) and X is a chalcogen atom (such as S, Se or Te) [21]. Considering their favorable electronic and mechanical properties, TMDCs are widely used for fundamental studies and applications in high-end electronics [22], separation [23], energy storage [24], electrochemical detection [25], and personalized medicine [26]. Furthermore, laminar membranes reconstructed from molybdenum disulfide (MoS2) nanosheets have recently shown great promise for water purification and desalination due to efficient salt rejection, high water fluxes, and long-term stability [27, 28].
However, to date, the state-of-the-art nacre-mimetic 2D layered materials compose of merely a single type of 2D nanobuilding-blocks, and thus, the mass and charge transport through such membranes are symmetric [9]. Fabrication of heterogeneous 2D layered membranes and realizing asymmetric transport behavior within such 2D nanofluidic systems become highly demanded and great challenging [29, 30].
In this context, herein, we report the fabrication of MoS2/WSe2 bi-layered membrane structure, and investigate the asymmetric ion transport properties therein. Through a two-step vacuumfiltration process (Scheme 1), WSe2 and MoS2 nanosheets were sequentially deposited onto a polymeric nanoporous support. Highly rectified ion transport phenomenon is observed through the WSe2/MoS2 2D bi-layered membranes. The preferential direction for ion transport is from the WSe2 layers to the MoS2 layers. The maximum ionic current rectification (ICR) ratio approaches 35 at intermediate electrolyte concentration. More intriguingly, by exchanging the deposition order of MoS2 and WSe2 layers, the observed ICR effect can be reversed, which justifies that the highly rectified ion transport phenomenon results from the asymmetry in the reconstructed 2D layered materials. The discovery of ICR effect in 2D nanofluidic heterostructures provides further opportunities for innovative nanofluidic devices and materials.

|
Download:
|
| Scheme 1. Construction of 2D bi-layered membranes. Through a two-step vacuum-filtration process, WSe2 and MoS2 nanosheets were successively deposited on a nanoporous support, forming the WSe2/MoS2 bilayer membrane. | |
MoS2 and WSe2 nanosheets were purchased from Nanjing MKNANO Tech. They were dispersed in a mixed solution of ethanol and water with the volume ratio of 1:1. Their lateral size is in the range of 0.5–5 μm. The dispersion can be stable at least for several weeks in common lab environment. Zeta potential measurements (Malvern Zetasizer, NanoZS90) show that the MoS2 and WSe2 colloids are negatively charged with zeta potentials of -32 mV and -39 mV, respectively. The purified MoS2 and WSe2 dispersions (0.05 mg/mL) can be stable for months (Fig. 1a).

|
Download:
|
| Fig. 1. Characterization of the supported WSe2/MoS2 bi-layered membrane. (a) Well dispersed MoS2 and WSe2 colloids (0.05 mg/mL). (b) A Photograph of the supported WSe2/MoS2 bi-layered membrane. (c) SEM observation on the crosssection of the bi-layered membrane shows laminar structure. (d) XRD patterns of WSe2/MoS2, MoS2, and WSe2 membranes. | |
To fabricate the membrane (Scheme 1), firstly, 40 mL WSe2 dispersion (0.25 mg/mL) was filtrated through a piece of polycarbonate membrane containing one-dimensional (1D) cylindrical nanopore array (mean pore size ~200 nm). The lateral size of the resulting membrane is about 25 mm. Afterward, 40 mL MoS2 dispersion (0.25 mg/mL) was deposited onto the WSe2 membrane through similar vacuum filtration process, yielding supported WSe2/MoS2 bilayer structure (Fig. 1b). The as-prepared membrane was dried in ambient condition for three days before further tests.
Scanning electron microscopic (SEM, Hitachi s-4800) observation on the cross-section of the WSe2/MoS2 bi-layered membrane clearly shows the laminar structure (Fig. 1c). The thickness of the bi-layered membrane is about 7 μm. Further X-ray diffraction (XRD, Bruker D8 focus) tests on the single-layered MoS2 and WSe2 membranes reveal diffraction peaks at 13.31° and 14.27°, corresponding to the interlayer spacing of 0.62 and 0.66 nm, respectively (Fig. 1d) [31, 32]. XRD measurement on the WSe2/MoS2 bi-layered membrane shows a combination of the two diffraction peaks, indicating the presence of the two-component membrane structure.
To investigate the ion transport properties through the reconstructed TMDCs multilayers, the tested membrane was mounted in between a two-compartment electrochemical cell, as described in detail in our previous works [33, 34]. The testing membrane area was about 0.2 mm2. The transmembrane ionic current was recorded with a pair of Ag/AgCl electrode connected to a Keithley 2636 B source meter. A scanning voltage from -2 V to +2 V was applied across the membrane with the reference potential set on the 1D nanoporous support. The electrolyte was 10 mmol/L KCl solution in each compartment.
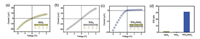
For the supported WSe2 or MoS2 single-layered membranes, the current-voltage responses become slightly rectified with ICR ratio not more than 2.0 (Figs. 2a and b). This phenomenon is due to the heterostructure between the 2D layered membrane and the beneath 1D nanopore array [35]. In sharp contrast, the supported WSe2/MoS2 bi-layered membranes exhibit very strong rectified current-voltage response with ICR ratio up to ~30 (Figs. 2c and d). The preferential direction for ion transport through the 2D bilayered membranes is from the WSe2 layers to the MoS2 layers.
|
Download:
|
| Fig. 2. Ion transport properties. (a, b) Supported single-layered MoS2 or WSe2 membranes show slight rectified ion transport behavior due to the heterostructures between the 2D layered membrane and the beneath 1D nanopore array. (c) WSe2/MoS2 bi-layered membranes show strong ICR effect. The ICR ratio can be up to 30. Their ICR ratios are summarized in (d). | |
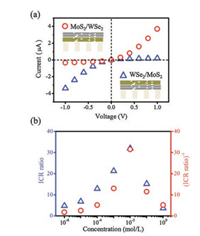
More intriguingly, by exchanging the deposition order of the MoS2 and WSe2 layers, strong ICR effect can be also found in the MoS2/WSe2 bi-layered membranes, but the direction of the I–V curve is totally reversed (Fig. 3a, the preferential direction is also from the WSe2 layers to the MoS2 layers). These evidences justify that the highly rectified ion transport phenomenon results from the asymmetry in the reconstructed 2D layered materials, rather than from the 1D/2D heterostructure [36]. Since the interlayer distance between the restacked WSe2 and MoS2 nanosheets, as well as the charge properties, are quite close to each other, the origin of the strong ICR effect found here needs further experimental and theoretical efforts to explore [37, 38].
|
Download:
|
| Fig. 3. Ionic rectification through bi-layered membranes. (a) By exchanging the deposition order of MoS2 and WSe2 layers, the resulting WSe2/MoS2 (blue triangle) and MoS2/WSe2 (red circle) bi-layered membranes show totally reversed currentvoltage responses. (b) The degree of ICR depends on the ionic concentration of the electrolyte solution. The maximum ICR ratio is found near an intermediate concentration of 10-2 mol/L. | |
Furthermore, the concentration-dependence of the ICR ratio is also investigated. From 10-6 mol/L to 1 mol/L, rectified ion transport properties are all found through the bi-layered membranes (Fig. 3b). A peak value of about 35 is found near an intermediate concentration of 10-2 mol/L. Both the WSe2/MoS2 and MoS2/WSe2 membrane structure share the trend. This observation is also in accord with that previously found in 1D nanofluidic systems [39, 40].
In conclusion, we fabricate heterogeneous 2D layered materials via sequential deposition of WSe2 and MoS2 nanosheets on support membranes, and investigate the asymmetric ion transport properties therein. Highly rectified current-voltage responses are found in the 2D bi-layered membrane structures. The preferential ion transport direction is from the WSe2 layers to the MoS2 layers. The asymmetries in chemical composition, interlayer distance, and surface charge properties between the two types of reconstructed 2D layered materials are responsible for the generation of strong ICR effects in the bi-layered membrane. From the fundamental aspects, the discovery of ICR effect in heterogeneous 2D nanofluidic systems further extend the range where this asymmetric ion transport phenomenon is found. More importantly, from the application aspects, the heterogeneous 2D layered membranes open up new possibilities for innovative 2D nanofluidic systems and materials [41].
AcknowledgmentsThis work dedicates to the 120th anniversary of Peking University, and is supported by the National Natural Science Foundation of China (Nos. 21522108, 11290163).
| [1] |
A.R. Koltonow, J. Huang, Science 351 (2016) 1395-1396. DOI:10.1126/science.aaf5289 |
| [2] |
Y. Feng, W. Zhu, W. Guo, L. Jiang, Adv. Mater. 29 (2017) 1702773. DOI:10.1002/adma.201702773 |
| [3] |
D. Li, R.B. Kaner, Science 320 (2008) 1170-1171. DOI:10.1126/science.1158180 |
| [4] |
W. Guo, L. Jiang, Sci. China Mater. 57 (2014) 2-6. DOI:10.1007/s40843-014-0005-z |
| [5] |
G. Liu, W. Jin, N. Xu, Angew. Chem. Int. Ed. 55 (2016) 13384-13397. DOI:10.1002/anie.201600438 |
| [6] |
J.R. Werber, C.O. Osuji, M. Elimelech, Nat. Rev. Mater. 1 (2016) 16018. DOI:10.1038/natrevmats.2016.18 |
| [7] |
H.B. Park, J. Kamcev, L.M. Robeson, M. Elimelech, B.D. Freeman, Science 356 (2017) eaab0530. DOI:10.1126/science.aab0530 |
| [8] |
B.E. Logan, M. Elimelech, Nature 488 (2012) 313-319. DOI:10.1038/nature11477 |
| [9] |
J. Gao, Y.P. Feng, W. Guo, L. Jiang, Chem. Soc. Rev. 46 (2017) 5400-5424. DOI:10.1039/C7CS00369B |
| [10] |
P. Sun, K. Wang, H. Zhu, Adv. Mater. 28 (2016) 2287-2310. DOI:10.1002/adma.201502595 |
| [11] |
M. Tsapatsis, AIChE J. 60 (2014) 2374-2381. DOI:10.1002/aic.v60.7 |
| [12] |
Y. Peng, Y. Li, Y. Ban, et al., Science 346 (2014) 1356-1360. DOI:10.1126/science.1254227 |
| [13] |
H. Cheng, Y. Zhou, Y. Feng, et al., Adv. Mater 29 (2017) 1700177. DOI:10.1002/adma.201700177 |
| [14] |
J. Gao, W. Guo, H. Geng, et al., Nano Res. 5 (2012) 99-108. DOI:10.1007/s12274-011-0189-7 |
| [15] |
Y. Jiang, J. Gao, W. Guo, L. Jiang, Chem. Commun. 50 (2014) 14149-14152. DOI:10.1039/C4CC06008C |
| [16] |
M. Chhowalla, H.S. Shin, G. Eda, et al., Nat. Chem. 5 (2013) 263-275. DOI:10.1038/nchem.1589 |
| [17] |
R. Lv, J.A. Robinson, R.E. Schaak, et al., Acc. Chem. Res. 48 (2015) 56-64. DOI:10.1021/ar5002846 |
| [18] |
Y. Hu, Y. Huang, C. Tan, et al., Mater. Chem. Front. 1 (2017) 24-36. DOI:10.1039/C6QM00195E |
| [19] |
Y. Ma, B. Li, S. Yang, Mater. Chem. Front. 2 (2018) 456-467. DOI:10.1039/C7QM00548B |
| [20] |
M. Zhang, C. Hou, A. Halder, H. Wang, Q. Chi, Mater. Chem. Front. 1 (2017) 37-60. DOI:10.1039/C6QM00145A |
| [21] |
S. Manzeli, D. Ovchinnikov, D. Pasquier, O.V. Yazyev, A. Kis, Nat. Rev. Mater. 2 (2017) 17033. DOI:10.1038/natrevmats.2017.33 |
| [22] |
B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti, A. Kis, Nat. Nanotechnol. 6 (2011) 147-150. DOI:10.1038/nnano.2010.279 |
| [23] |
Y. Wang, C. Wang, Chin. Chem. Lett. 29 (2018) 345-352. DOI:10.1016/j.cclet.2018.01.001 |
| [24] |
H. Yuan, L. Kong, T. Li, Q. Zhang, Chin. Chem. Lett. 28 (2017) 2180-2194. DOI:10.1016/j.cclet.2017.11.038 |
| [25] |
H.Y. Chen, J. Wang, L. Meng, T. Yang, K. Jiao, Chin. Chem. Lett. 27 (2016) 231-234. DOI:10.1016/j.cclet.2015.09.018 |
| [26] |
H. Wang, Z. Lu, S. Xu, et al., Proc. Natl. Acad. Sci. U. S.A. 110 (2013) 19701-19706. DOI:10.1073/pnas.1316792110 |
| [27] |
L. Sun, H. Huang, X. Peng, Chem. Commun. 49 (2013) 10718-10720. DOI:10.1039/c3cc46136j |
| [28] |
W. Hirunpinyopas, E. Prestat, S.D. Worrall, et al., ACS Nano 11 (2017) 11082-11090. DOI:10.1021/acsnano.7b05124 |
| [29] |
W. Guo, Y. Tian, L. Jiang, Acc. Chem. Res. 46 (2013) 2834-2846. DOI:10.1021/ar400024p |
| [30] |
L. Wang, Y. Feng, Y. Zhou, et al., Chem. Sci. 8 (2017) 4381-4386. DOI:10.1039/C7SC00153C |
| [31] |
Z. Wang, Q. Tu, S. Zheng, et al., Nano Lett. 17 (2017) 7289-7298. DOI:10.1021/acs.nanolett.7b02804 |
| [32] |
M. Deng, K. Kwac, M. Li, Y. Jung, H.G. Park, Nano Lett. 17 (2017) 2342-2348. DOI:10.1021/acs.nanolett.6b05238 |
| [33] |
W. Guo, C. Cheng, Y. Wu, et al., Adv. Mater. 25 (2013) 6064-6068. DOI:10.1002/adma.201302441 |
| [34] |
J. Ji, Q. Kang, Y. Zhou, et al., Adv. Funct. Mater. 27 (2017) 1603623. DOI:10.1002/adfm.v27.2 |
| [35] |
J. Gao, W. Guo, D. Feng, et al., J. Am. Chem. Soc. 136 (2014) 12265-12272. DOI:10.1021/ja503692z |
| [36] |
L.J. Cheng, L.J. Guo, ACS Nano 3 (2009) 575-584. DOI:10.1021/nn8007542 |
| [37] |
Y. Jiang, Y. Feng, J. Su, et al., J. Am. Chem. Soc. 139 (2017) 18739-18746. DOI:10.1021/jacs.7b11732 |
| [38] |
L.X. Cao, F.L. Xiao, Y.P. Feng, et al., Adv. Funct. Mater 27 (2017) 1604302. DOI:10.1002/adfm.201604302 |
| [39] |
Z.S. Siwy, Adv. Funct. Mater. 16 (2006) 735-746. DOI:10.1002/(ISSN)1616-3028 |
| [40] |
W. Guo, J. Xue, L. Wang, Y. Wang, Nucl. Instrum. Meth. B 266 (2008) 3095-3099. DOI:10.1016/j.nimb.2008.03.169 |
| [41] |
J. Gao, A. R. Koltonow, K. Raidongia, et al., Mater. Chem. Front. 2 (2018) 475-482. DOI:10.1039/C7QM00620A |
 2018, Vol. 29
2018, Vol. 29 


