b Department of Materials Science and Engineering, National University of Singapore, Singapore 117575, Singapore;
c Institute of Microelectronics, A*STAR(Agency for Science, Technology and Research), Singapore 138634, Singapore
Two-dimensional (2D) layered materials, such as graphene and graphene oxide [1], transition metal dichalcogenides (TMDs) [2], carbon nitrides (C3N4) [3], and metal oxide [4], featuring thicknesses down to atomic/molecular level with lateral sizes up to micrometer range, have attracted increasing attentions recently due to their unique physical and chemical properties distinct from those of their bulk counterparts [5]. For instance, single layer graphene possesses exceptional electric, thermal, mechanical and optical properties, and thereby shows great potentials in a variety of applications, including energy storing devices, solar cells, photocatalytic systems, etc. [6-8]. These exotic properties of 2D materials prompt the discovery of their organic counterparts, which may largely broaden the family of 2D materials because of their infinite possibilities in molecular and topological designs. Covalent organic frameworks (COFs) are crystalline polymers composed of light elements connected by covalent bonds into 2D or 3D frameworks [9]. Recently, covalent organic nanosheets (CONs), originated from COFs with 2D structures, have been reported as organic analogues to the conventional inorganic 2D materials [10]. CONs possess many promising features, such as tunable pore size, adjustable skeleton geometry and designable functionality.
Generally speaking, there are two different strategies that can be used to obtain CONs: top-down and bottom-up. The top-down strategy involves the exfoliation of bulk COF powders through mechanical delamination [10] or chemical reaction [11], while bottom-up one refers to the growth of CONs directly from small molecular building blocks with desired 2D structures. The topdown strategy relies heavily on the crystal size of bulk COFs, while the growth of large COF crystals still remains as a big challenge [12]. As a result, the exfoliated CONs possess lateral sizes typically within nanometer range, which are still far from the micrometer requirement for device fabrication. In addition, strong π-π interlayer stacking interactions in bulk COFs and possible restacking processes make it difficult to obtain ultrathin CONs with high aspect ratios.
On the contrary, the bottom-up strategy can theoretically produce CONs with atomic thicknesses and unlimited lateral sizes. Abel et al. reported the first example of direct synthesis of monolayered CONs, in which boronate monomers were deposited onto Au (111) surface and the polymerization reaction proceeded under ultrahigh vacuum conditions [13]. Subsequently, Loh et al. produced a monolayer of rectangular-grid network consisted of strong C--C covalent bonds [14]. However, these synthesized monolayered CONs suffer from defects and small lateral sizes. Alternatively, King et al. reported the synthesis of 2D polymers through the photopolymerization of anthraceno-based amphiphilic monomers at the air-water interface on a Langmuir-Blodgett (LB) trough [15]. The honeycomb lattice observed under scanning tunnelling microscopy (STM) reveals a good in-plane order in this 2D polymer. Following this strategy, Zhang et al. adopted selfcorrected dynamic imine chemistry at the air-water interface, and obtained free-standing 2D covalent organic monolayers exhibiting a lateral size up to tens of micrometers [16]. However, the crystallinity of their monolayers remains unclear, which hampers the deep understanding of these materials. Until very recently, Feng et al. synthesized wafer-sized monolayered CONs not only exhibiting good crystallinity, but also possessing a mechanical strength on the same order of that of graphene [17].
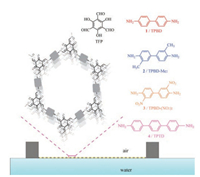
Schiff base linkages are widely used in the preparation of COFs, and the enol-to-keto tautomerization in Schiff base COFs can give them excellent stability toward water, acid and even base [18]. Conventional solvothermal reactions in sealed vials afford microcrystalline COF powders, which need further exfoliation under prolonged sonication to prepare CONs [19]. Herein, we demonstrate that chemically stable CONs with adjustable hydrophobicity can be prepared at the air-water interface on a LB trough with short reaction time and mild synthetic condition. Briefly, 10 μL of 1, 3, 5-triformylphloroglucinol (TFP, 1mg/mL) in an equimolar mixture of chloroform and N, N-dimethylformamide was dropped and spread to form a densely packed layer at the airwater interface, followed by the addition of monomer 1-4 (all in 1mg/mL) to produce TPBD, TPBD-Me2, TPBD-(NO2)2 and TPTD CONs, respectively (Scheme 1). The isotherm of the surface pressure (SP) versus the mean molecular area (MMA) was recorded for TPBD before and after the polymerization (Fig. S1 in Supporting information). The presence of a sharp increase in the SP-MMA isotherm after the polymerization suggests the compression on nanosheets by barriers of the LB trough. The polymerization process was monitored by the atomic force microscopy (AFM) analysis on TPBD CONs deposited on SiO2/Si wafer after different reaction time. Separated aggregates appeared after 4h, which gradually evolved into interconnected nanosheets with large voids (after 8h), continuous nanosheets with minor defects (after 12h), and eventually smooth and coherent nanosheets (after 24h, Fig. S2 in Supporting information). More interestingly, after 24h of polymerization, the large and defect-free TPBD CONs floating at the air-water interface can even be recorded by the photography (Fig. S3 in Supporting information). Inspired by this finding, we performed the synthesis of other three CONs under similar conditions (for synthetic procedures, see Experimental section in Supporting information), and transferred all CONs onto different substrates for further characterizations, namely, SiO2/Si wafer for AFM, optical microscopy (OM) and Raman microscopy analysis, copper grid for transmission electron microscopy (TEM), and clean glass slide for X-ray spectroscopy (XPS).
|
Download:
|
| Scheme 1. Chemical structures of monomers and schematic illustration of CONs synthesized at the air-water interface by Langmuir-Blodgett method. | |
The smooth surface of all CONs are revealed by AFM measurements in a tapping mode, with thicknesses ranging from 4nm to 10nm corresponding to 12–30 single layers (Fig. 1, column 2). TEM analyses further confirm the sheet-like morphology of these four CONs with fractures possibly originating from the sample transfer process (Fig. 1, column 3). Wrinkles and folds can be frequently observed in all CONs under TEM, illustrating the flexibility of these nanosheets. High resolutionTEM (HRTEM) imagesindicate distinct lattice fringes due to the good crystallinity of these CONs. The lattice spacing ranges from 0.32nm to 0.36nm, suggesting the (001) crystalline facet. The crystallinity of CONs was further investigated by selected area electron diffraction (SAED), revealing hexagonal patterns for TPBD and TPTD CONs.However, the SAED of TPBD-Me2 and TPBD-(NO2)2 CONs onlydisplays randomlyoriented patterns, which could be attributed to the disturbance of the interlayer stacking caused by the introduction of functional groups into these two CONs. This also helps to explain the lower crystallinity and surface area of their corresponding COFs [10]. The OM images clearly disclose the presence of CONs deposited on SiO2/Si wafer with areas even larger than 200 μm ×200 μm (Fig. 1, column 4). Therefore, the aspect ratio of these CONs can exceed 20 000, surpassing that of all other CONs (~300–500) obtained from liquid-assisted exfoliation or chemical delamination [11, 20].

|
Download:
|
| Fig. 1. Crystal structures, AFM, TEM, OM images and N 1s XPS spectra of CONs and their corresponding COFs. Inserted in TEM images are HRTEM images (upper right) and SAED patterns (bottom right). | |
The elemental composition and chemical bonding of all CONs and their corresponding bulk COFs were analysed by X-ray spectroscopy (XPS). As shown in Fig. 1, columns 5 and 6, the N1s XPS signal of all CONs and COFs in the binding energy range of 398–402eV can be deconvoluted intothree parts, correspondingto imine nitrogen (C=N), unreacted amine groups (Ph–NH2) at domain edges or defects in the crystals, and pyrrole nitrogen (C—N) in the central part of the crystals due to the irreversible enol-to-keto tautomerization [18]. The extra N1s peak at ~406eV in TPBD-(NO2)2 can be attributed to the --NO2 groups from its building blocks. Interestingly, we observed a minor reduction in N 1s binding energy for all CONs compared to COFs (399.8 versus 400.1eV for TPBD, 399.6 versus 400.1eV for TPBD-Me2, 399.4 versus 400.4eV for TPBD-(NO2)2 and 399.7 versus 400.4eV for TPTD). This isas expected because the ultrathin featureof theCONs weakens their interlayer interactions and reduces the electron cloud density of nitrogen atoms [21]. Similar phenomenon has been reported in the exfoliation of boron-based COFs down to nanometer thickness [20].
To gain further insight into the chemical structure of the obtained CONs, Raman spectroscopy was adopted here due to its atomic level sensitivity in probing chemical linkages in thin film structures. We investigated Raman spectra of all CONs, COFs and their monomers by measuring vibrations in bands corresponding to the C—N, C=N, CHO and Ph–NH2 groups (Fig. 2). In the Raman spectrum of TFP, the band at 1642cm-1 can be attributed to the C=O stretching in aldehyde groups [16]. Meanwhile, the N--H bands belonging to Ph–NH2 groups in monomer 1, 2 and 4 locate at 1144, 1316 and 1281cm-1, respectively [17]. Both bands disappear in the spectra of all COFs and CONs, demonstrating the complete reaction of aldehyde and amine groups after polymerization. The newly formed C—N and C=N bonds at ~1100–1180 and ~1600cm-1 are clearly visible in the Raman spectra of all CONs and COFs except TPBD-(NO2)2. The exact reason for the lack of C—N and C=N peaks inTPBD-(NO2)2 remains unclear to us, but we speculate that the existence of strong electro-withdrawing nitro groups in its building block may weaken the Raman intensity of the whole structure of TPBD-(NO2)2 [22]. Consequently, both TPBD- (NO2)2 CONs and COFs show the prominent peaks at ~820 and ~1350 cm-1 characteristic for -NO2 stretching. All CONs investigated in this study showed similar Raman spectra compared to their corresponding COFs, revealing the same chemical bonds inside these two different types of structures.

|
Download:
|
| Fig. 2. Raman spectra of CONs, COFs and their corresponding building blocks: (a) TPBD; (b) TPBD-Me2; (c) TPBD-(NO2)2; (d) TPTD. | |
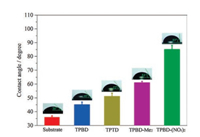
Recently, COFs have been demonstrated to possess great potentials in chemically stable super-hydrophobic coatings [23]. The large lateral size and variable organic building blocks of assynthesized CONs motivate us to evaluate their performance in surface coatings. All CONs were vertically transferred onto SiO2/Si wafers, covering the whole substrate and forming the surface coatings. Water contact angle measurements indicated a contact angle of 36° for the bare substrate (Fig. 3), which is due to the formation of hydrogen bonding networks at SiO2-water interfaces [24]. Water contact angles of the surface coatings increase in the order of TPBD (45°) < TPTD (52°) < TPBD-Me2 (61°) < TPBD-(NO2)2 (85°), revealing the tunable hydrophobicity of these CONs. Higher benzene ring density in TPTD and pendant methyl groups in TPBDMe2 or nitro groups in TPBD-(NO2)2 lead to the enhancement in hydrophobicity compared to original TPBD. This corresponds well to the water vapour uptake in these CONs obtained via mechanical exfoliation [10]. We further transferred TPBD CONs onto the porous polyethersulfone support and they could cover the whole support without any visible defect (Fig. S5 in Supporting information). This paves the way for utilizing CONs as membrane materials for molecular separations.
|
Download:
|
| Fig. 3. Water contact angles of CONs on SiO2/Si substrate. | |
In conclusion, we have successfully fabricated four CONs at the air-water interface by LB method. The expected chemical compositions of prepared CONs were confirmed by XPS and Raman spectroscopy. In addition, CONs prepared by LB method in this study retain their crystallinity with an ultrasmall thickness of around 10 nm and large lateral sizes up to 200 μm, indicating aspect ratios higher than 20 000 which are hard to achieve in conventional top-down exfoliation processes. This work offers the platform of designing and preparing novel functional CONs with large lateral size and high aspect ratio suitable for device fabrications with promising applications in separation, sensing, catalysis, etc.
AcknowledgmentsThis work is supported by National University of Singapore (No. CENGas R-261-508-001-646) and Ministry of Education – Singapore (No. MOE AcRF Tier 1 R-279-000-472-112).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.09.002.
| [1] |
K.S. Novoselov, A.K. Geim, S.V. Morozov, et al., Science 306 (2004) 666-669. DOI:10.1126/science.1102896 |
| [2] |
M. Chhowalla, Z. Liu, H. Zhang, Chem. Soc. Rev. 44 (2015) 2584-2586. DOI:10.1039/C5CS90037A |
| [3] |
J. Zhang, Y. Chen, X. Wang, Energy Environ. Sci. 8 (2015) 3092-3108. DOI:10.1039/C5EE01895A |
| [4] |
Z. Huang, A. Zhou, J. Wu, et al., Adv. Mater. 28 (2016) 1703-1708. DOI:10.1002/adma.201504484 |
| [5] |
M. Xu, T. Liang, M. Shi, H. Chen, Chem. Rev. 113 (2013) 3766-3798. DOI:10.1021/cr300263a |
| [6] |
Y. Zhu, S. Murali, M.D. Stoller, et al., Science 332 (2011) 1537-1541. DOI:10.1126/science.1200770 |
| [7] |
J.H. Chen, C. Jang, S. Xiao, M. Ishigami, M.S. Fuhrer, Nat. Nanotechnol. 3 (2008) 206-209. DOI:10.1038/nnano.2008.58 |
| [8] |
Q. Li, B. Guo, J. Yu, et al., J. Am. Chem. Soc. 133 (2011) 10878-10884. DOI:10.1021/ja2025454 |
| [9] |
P.J. Waller, F. Gándara, O.M. Yaghi, Acc. Chem. Res. 48 (2015) 3053-3063. DOI:10.1021/acs.accounts.5b00369 |
| [10] |
S. Chandra, S. Kandambeth, B.P. Biswal, et al., J. Am. Chem. Soc. 135 (2013) 17853-17861. DOI:10.1021/ja408121p |
| [11] |
M.A. Khayum, S. Kandambeth, S. Mitra, et al., Angew. Chem. Int. Ed. 55 (2016) 15604-15608. DOI:10.1002/anie.201607812 |
| [12] |
A.P. Côté, A.I. Benin, N.W. Ockwig, et al., Science 310 (2005) 1166-1170. DOI:10.1126/science.1120411 |
| [13] |
N.A.A. Zwaneveld, R. Pawlak, M. Abel, et al., J. Am. Chem. Soc. 130 (2008) 6678-6679. DOI:10.1021/ja800906f |
| [14] |
W. Liu, X. Luo, Y. Bao, et al., Nat. Chem. 9 (2017) 563-570. DOI:10.1038/nchem.2696 |
| [15] |
D.J. Murray, D.D. Patterson, P. Payamyar, et al., J. Am. Chem. Soc. 137 (2015) 3450-3453. DOI:10.1021/ja512018j |
| [16] |
W. Dai, F. Shao, J. Szczerbiński, et al., Angew. Chem. Int. Ed. 128 (2016) 221-225. DOI:10.1002/ange.201508473 |
| [17] |
H. Sahabudeen, H. Qi, B.A. Glatz, et al., Nat. Commun. 7 (2016) 13461. DOI:10.1038/ncomms13461 |
| [18] |
S. Kandambeth, A. Mallick, B. Lukose, et al., J. Am. Chem. Soc. 134 (2012) 19524-19527. DOI:10.1021/ja308278w |
| [19] |
Z. Kang, Y. Peng, Y. Qian, et al., Chem. Mater. 28 (2016) 1277-1285. DOI:10.1021/acs.chemmater.5b02902 |
| [20] |
I. Berlanga, M.L. Ruiz-González, J.M. González-Calbet, et al., Small 7 (2011) 1207-1211. DOI:10.1002/smll.v7.9 |
| [21] |
B. Lukose, A. Kuc, T. Heine, Chem. Eur. J. 17 (2011) 2388-2392. DOI:10.1002/chem.201001290 |
| [22] |
H. Zhou, Z. Zhang, C. Jiang, et al., Anal. Chem. 83 (2011) 6913-6917. DOI:10.1021/ac201407z |
| [23] |
D. Mullangi, S. Shalini, S. Nandi, B. Choksi, V. Ramanathan, J. Mater. Chem. A 5 (2017) 8376-8384. DOI:10.1039/C7TA01302G |
| [24] |
P.K. Chow, E. Singh, B.C. Viana, et al., ACS Nano 9 (2015) 3023-3031. DOI:10.1021/nn5072073 |
 2018, Vol. 29
2018, Vol. 29 


