Accurate measurements of temperature within the physiological range are of great importance for understanding physiological/pathological processes, preventing and treating cancers, and controlling drug release systems [1]. On the one hand, temperature affects many physiological processes ranging from gene expression to energy metabolism and it varies from cells to cells depending on their different functionalized biochemical reactions. As such, data on cellular temperatures can provide important insights into physiological processes [2]. On the other hand, the appearance of thermal singularities is one of the first signatures of many diseases such as inflammation, cancer, and cardiac problems. In disease states such as cancer, malignant cells commonly are at elevated temperature than healthy cells [3]. Consequently, it is important to develop techniques that enable reliable and accurate detecting of temperature within the physiological range [4]. To this end, luminescence-based temperature sensors have shown great potential since their non-invasiveness, accuracy, fast response, inertness to strong electromagnetic fields, and low-damage to tissues [5].
Metal-organic frameworks (MOFs), self-assembled from metal cations or clusters and organic linkers, have potential advantages as novel platforms for the development of solid state luminescent thermometer [6]. Not only the building components of MOFs, metal centers, organic linkers, and guest molecules but also the energy-exchange processes and pathways among these building moieties can generate temperature-dependent luminescence emission [7]. MOF thermometers based on temperature-dependent energy transfer such as Tb3+-to-Eu3+ [8], Nd3+-to-Yb3+ [9], ligand-to-Eu3+/Tb3+ [10], dye-to-Eu3+/Tb3+ [11], ligand-to-ligand [12], and dye-to-ligand [13] have been achieved in working range from cryogenic to physiological temperature, and to moderately high temperature. However, MOF-based thermometers working in physiological temperature range are quite rare and the reported thermometers often suffer from a low relative sensitivity [14]. It is still a great challenge to exploit MOF-based thermometers with higher sensitivity [15].
The luminescence of Ln3+ in lanthanide MOFs depends on the energy transfer (EnT) from organic ligands to metals, that is "antenna effect". When the energy gap (ΔE) between the ligand's lowest triplet level (T1) and the Ln3+ lowest emitting level is proper, the back energy transfer (BEnT) from Ln3+ ion to ligand might occur [16]. The BEnT is temperature-dependent and needs thermal activation energy (ΔE). A strong BEnT will take place when temperature is high enough to provide activation energy ΔE, resulting in a strong quenching of Ln3+ emission. Based on this theory, Carlos et al. presented a single europium MOF luminescent thermometer [17]. The small energy difference between the T1 and 5D0 levels of Eu3+ (ΔE = 553 cm-1) allows efficient cryogenic measurement, but the relative sensitivity is very poor in the physiological temperature range (0.33% K-1 at 300 K). Herein, we reported a Eu3+/Gd3+-mixed strategy to improve the relative sensitivity of BEnT-based thermometer. This strategy is employed for the following considerations: 1) The excited-state energy level of Gd3+ ion is high enough that the ligand cannot sensitize its luminescence. When doping appropriate content of Eu3+ ion, both the emissions from ligand and Eu3+ ion will be obtained simultaneously, which allows the ratiometric and colorimetric temperature sensing; 2) On average, there are more ligands around each Eu3+ ion as acceptor in the mixed MOFs than the pure europium MOF, which will enhance BEnT from Eu3+ ion to ligand, thereby improving the sensitivity. The resultant Eu0.0005Gd0.9995NDC exhibits highly temperature-dependent emission, exceptional stability and good biocompatibility, and can detect physiological temperature with single-emission, dualemission, and colorimetric models.
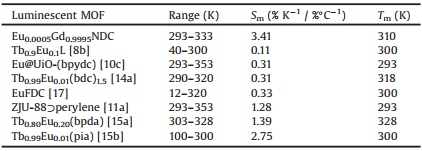
The polyhedral crystals of LnNDC (Ln = Eu3+, Gd3+) were synthesized according to previous literature [18]. This MOF crystallizes in the cubic space group Fm3m, and adopts the hexanuclear metal-cluster [Ln6(μ3-OH)8(O2C-)12] as secondary building units (SBUs) and organic NDC2- as bridging ligand to form a three-dimensional framework (Fig. 1). The mixed-lanthanide MOFs Eu0.01Gd0.99NDC, Eu0.003Gd0.997NDC, Eu0.001Gd0.999NDC, and Eu0.0005Gd0.9995NDC were obtained by varying the molar ratio of Eu (NO3)3·6H2O and Gd(NO3)3·6H2O in the starting reactants. Eu3+ ions are randomly substituted for the position of Gd3+ ions and evenly distributed in the resulting MOFs, and the molar ratios of Eu3+/Gd3+ were confirmed by inductively coupled plasma atomic emission spectroscopy (ICP) (Table S1 in Supporting information). The phase purity of these MOFs were independently confirmed by powder X-ray diffraction (PXRD), Fourier transform infrared spectroscopy (FTIR), and thermogravimetric analyses (TGA) (Figs. S1-S3 in Supporting information).
|
Download:
|
| Fig. 1. Crystal structure of EuNDC viewed along (101) direction, C, black; O, red; Eu, green; all H atoms and solvent molecules are omitted for clarity. The blue polyhedras represent the hexanuclear metal-cluster SBUs and the organic ligands NDC2- are simplified as black lines. | |
The excitation and emission spectra of powdered Eu0.0005Gd0.9995NDC were recorded at room temperature (Fig. S4 in Supporting information). When monitoring the Eu3+5D0 → 7F2 transition at 614 nm, an intense and broad band with a maximum at around 335 nm was observed, which is attributed to the NDC2- ligand π-π* electron transition. Upon excitation at 335 nm, Eu0.0005Gd0.9995NDC exhibits both the emission of the NDC2- ligands at about 400 nm and the characteristic transitions (5D0 → 7FJ, J = 0, 1, 2, 3, 4) of Eu3+ at 579, 594, 614, 652, and 701 nm. The relative emission intensity of NDC2- and Eu3+ can be easily adjusted by varying the doped amount of Eu3+. When increasing the doped content of Eu3+ up to 1%, the emission band of the ligand is very weak in the luminescence spectra and completely disappears in single europium MOF EuNDC. MellerBuschbaum and Heine demonstrated that a suitable energy difference between the T1 of the ligand and the excited lanthanide states is critical for efficient energy transfer (EnT) from ligand to lanthanide ions, which is about 2500–3500 cm-1. A smaller energy difference allows a partial back energy transfer (BEnT) of excitation energy from the metal to the ligand [19]. Increasing the temperature enhances the BEnT, resulting in a weaker Ln3+ emission. The T1 of NDC2- estimated from the 10 K phosphorescence spectra of GdNDC is 20161 cm-1 (Fig. S5 in Supporting information). The T1 of NDC2- is very close to the emitting level of 5D0 (17200 cm-1) of Eu3+, indicating that EuxGd1-xNDC can act as a luminescent thermometer based on BEnT.
The temperature-dependent emission properties of Eu0.0005Gd0.9995NDC were studied in physiological temperature range (Fig. 2). For comparison, the emission spectra of EuNDC were recorded under the same conditions (Fig. S6 in Supporting information). With the increasing temperature from 20 ℃ to 60 ℃, the luminescence intensity of Eu3+ in Eu0.0005Gd0.9995NDC shows a dramatic quenching about 83.4% while only 33.6% quenching is observed in EuNDC. The different quenching efficiency probably because there are more ligands around Eu3+ ion as acceptor in Eu0.0005Gd0.9995NDC, which is beneficial for BEnT from Eu3+ ion to NDC2-. The the lifetime of Eu3+ in EuNDC is longer than that in Eu0.0005Gd0.9995NDC in the whole temperature range, indicating the dilute Eu3+ is beneficial for BEnT (Fig. S7 in Supporting information). The ligand emission of Eu0.0005Gd0.9995NDC decreases slowly and quenches about 45.7%, indicating that Eu0.0005Gd0.9995NDC can be an outstanding thermometer in single-emission, dual-emission and colorimetric models.

|
Download:
|
| Fig. 2. (a) Temperature-dependent emission spectra of Eu0.0005Gd0.9995NDC in the physiological temperature range from 20 ℃ to 60 ℃. (b) Temperature-dependent integrated intensity and the fitting curve of single Eu3+. (c) Temperature-dependent integrated intensity ratio of Eu3+ to ligand and the fitting curve. (d) CIE chromaticity diagram showing the temperature-dependent luminescence color of Eu0.0005Gd0.9995NDC. | |
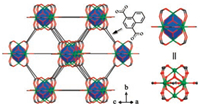
We first evaluated the temperature detection performance of Eu0.0005Gd0.9995NDC in single-emission model with Eu3+5D0 → 7F2 transition (IEu) as thermometric parameter. As shown in Fig. 2b, the integrated intensity of 5D0 → 7F2 transition from 605 nm to 625 nm decreases exponentially with increasing temperature. We adopted an empirical sigmoidal Boltzmann Origin8.0 built-in function (Eq. (1)) to fit the experimental calibration curve. There is a good fitting correlation with R2 = 0.9983 and other fitting parameters were presented in Table S2 (Supporting information).
s
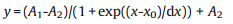
|
(1) |
To compare the performances of this and other reported thermometers, the relative sensitivity (Sr) is utilized and defined as Eq. (2):

|
(2) |
where IEu is the measured temperature-sensitive parameter and T is temperature. According to fitting results and Eq. 2, the relative sensitivity Sr of Eu0.0005Gd0.9995NDC in single-emission model enhances from 4.48% ℃-1 at 20 ℃ to the maximum 5.19% ℃-1 at 33 ℃ and then decreases to 2.55% ℃-1 at 60 ℃ (Fig. 3a).

|
Download:
|
| Fig. 3. Relative sensitivity (a) and temperature uncertainty (b) of Eu0.0005Gd0.9995NDC in single-emission and dual-emission models. | |
Luminescent thermometer based on single emission whose accuracy is often affected by luminophore quantity, excitation power, probe concentration, and the drifts of optoelectronic systems. Ratiometric thermometer based on dual-emission from two different transitions can make measuring results independent of external factors and allow accurate temperature readout. In Eu0.0005Gd0.9995NDC, the luminescence emission generated from NDC2- can be used as inner-temperature reference. We further utilized the integrated intensity ratio of 5D0 → 7F2 transition (IEu) to ligand's emission (INDC) as thermometric parameter Δ(Δ = IEu/INDC, IEu and INDC are defined as the integrated intensities of Eu3+ emission in the range of 605–625 nm and NDC2- emission in 360– 500 nm range, respectively). As shown in Fig. 2c, a good fitting relationship between Δ and temperature can be obtained with the above Eq. (1) (R2 = 0.9995, other fitting parameters were presented in Table S2 in Supporting information). The calculated relative sensitivity Sr of Eu0.0005Gd0.9995NDC in dual-emission model shows the same trend as single-emission model. The maximum relative sensitivity Sm = 3.41% ℃-1 is obtained at 37 ℃, which is higher than most of the other reported luminescent MOF thermometers working in the physiological temperature range (Table 1). The relative sensitivity can maintain above 3.00% ℃-1 in the whole physiological temperature range (Fig. 3a). Sm occurs in the physiological range probably because the physiological temperature is the well-matched temperature to provide thermal activation energy (ΔE) for BEnT. Proceeding from this point, it is easy to obtain a high-sensitive BEnT-based thermometer in targeted temperature section by the judicious choice of organic ligand with suitable T1. Thermometers working in a higher temperature might need a higher T1 and a lower T1 might indicate Sm occurs in a lower temperature.
|
|
Table 1 Comparison of maximum relative sensitivity (Sm, % K-1 / %℃-1) of other reported ratiometric luminescent MOF thermometers with ours working in the physiological temperature range. |
Temperature resolution (or temperature uncertainty) is another important factor for thermometer, which is evaluated by the following equation:
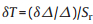
|
(3) |
where δT is the temperature resolution, δΔ/Δ is the relative error in the determination of the thermometric parameter. For commercially available fluorescence spectrometer, δΔ/Δ can be accurate to 0.02%. Under these circumstances, the temperature resolution of Eu0.0005Gd0.9995NDC is estimated to 0.004 ℃ and 0.006 ℃ at 37 ℃ for single-emission and dual-emission models, respectively. It is a splendid temperature resolution compared with other techniques, such as wired thermistors (0.01 ℃) and noncontact infrared cameras (1.0 ℃) [20].
The distinct color emission of Eu0.0005Gd0.9995NDC in different temperature makes it a sensitive colorimetric thermometer for in situ temperature measurement and thermal imaging. Based on the Commission Internationale de L'Eclairage (CIE 1931) chromaticity diagram coordinates transformed from the temperature-dependent emission spectra, the luminescence color of Eu0.0005Gd0.9995NDC gradually shifts from purplish-red at 20 ℃ to reddish-purple at 40 ℃, then to purple at 60 ℃ (Fig. 2d). According to the observed color with the naked eyes or a camera, the targeted temperature can be directly estimated by comparison with the CIE chromaticity diagram, thus providing a convenient approach for the monitoring of temperature distribution.
The temperature-dependent luminescence properties of Eu0.01Gd0.99NDC, Eu0.003Gd0.997NDC, and Eu0.001Gd0.999NDC were also investigated (Figs. S8-S10 in Supporting information). The temperature can also be exponentially related to IEu and IEu/INDC, suggesting the universal applicability of EuxGd1-xNDC for physiological temperature sensing. All of the Eu/Gd-mixed EuxGd1-xNDC thermometers exhibit a high relative sensitivity in physiological temperature range (Figs. S11 and S12 in Supporting information) and the temperature-responsive color section can be tuned by varying Eu3+ content.
To establish its potential for biological applications, we further investigated the stability and cytotoxicity of Eu0.0005Gd0.9995NDC under physiological conditions. Eu0.0005Gd0.9995NDC exhibits exceptional stability in water, acidic (pH 3.00), basic (pH 11.00), and phosphate buffered saline (PBS) solution as being certified by the PXRD (Fig. S13 in Supporting information). Furthermore, the cytotoxicity of Eu0.0005Gd0.9995NDC toward rat pheochromocytoma (PC12) cells was evaluated by the 3-(4, 5-dimethyl-2-thiazolyl)- 2, 5-diphenyltetrazolium bromide (MTT) assay. Eu0.0005Gd0.9995NDC has no obvious effects on cell viability even the dose concentration increased to 100 μg/mL (Fig. S14 in Supporting information), demonstrating its low toxicity and good biocompatibility.
In conclusion, by the judicious choice of organic ligand, we synthesized a family of Eu/Gd-mixed metal-organic frameworks EuxGd1-xNDC for multipath temperature readout in the physiological range. The suitable high triplet level of H2NDC and mixed Gd3+ dramatically weaken the emission of Eu3+ through thermally activated back energy transfer, thus allowing an ultrasensitive thermometer. Moreover, Eu0.0005Gd0.9995NDC exhibits good stability and biocompatibility, which can be a potential thermometer in biological sensing. This work would enlighten more research about the design of MOF-based thermometers with enhanced sensitivity in targeted temperature section.
AcknowledgmentThis work was financially supported by the National Natural Science Foundation of China (Nos. 51432001, 51472217, 51632008, U1609219, and 51772268).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.10.038.
| [1] |
(a) X. D. Wang, O. S. Wolfbeis, R. J. Meier, Chem. Soc. Rev. 42(2013) 7834-7869; (b) E. N. Ceron, D. H. Ortgies, B. Rosal, et al., Adv. Mater. 27(2015) 4781-4787; (c) H. Liu, Y. Fan, J. Wang, et al., Sci. Rep. 5(2015) 14879. |
| [2] |
S. Kalytchuk, K. Polakova, Y. Wang, et al., ACS Nano 11 (2017) 1432-1442. DOI:10.1021/acsnano.6b06670 |
| [3] |
F. Ye, C. Wu, Y. Jin, et al., J. Am. Chem. Soc. 133 (2011) 8146-8149. DOI:10.1021/ja202945g |
| [4] |
(a) Z. Chen, K. Y. Zhang, X. Tong, et al., Adv. Funct. Mater. 26(2016) 4386-4396; (b) H. Peng, M. I. Stich, J. Yu, et al., Adv. Mater. 22(2010) 716-719. |
| [5] |
(a) Z. Antic, M. D. Dramicanin, K. Prashanthi, et al., Adv. Mater. 28(2016) 7745-7752; (b) X. Wang, O. S. Wolfbeis, R. J. Meier, Chem. Soc. Rev. 42(2013) 7834-7869; (c) Y. Guo, S. Gu, X. Feng, et al., Chem. Sci. 5(2014) 4388-4393. |
| [6] |
(a) Y. Cui, F. Zhu, B. Chen, G. Qian, Chem. Commun. 51(2015) 7420-7431; (b) Y. Cui, B. Chen, G. Qian, Coord. Chem. Rev. 273-274(2014) 76-86; (c) P. Mahata, S. K. Mondal, D. K. Singha, P. Majee, Dalton Trans. 46(2017) 301-328; (d) T. L. Hu, H. Wang, B. Li, et al., Nat. Commun. 6(2015) 7328; (e) Y. Hu, M. Ding, X. Q. Liu, L. B. Sun, H. L. Jiang, Chem. Commun. 52(2016) 5734-5737; (f) Z. Hu, Y. Peng, Y. Gao, et al., Chem. Mater. 28(2016) 2659-2667; (g) Y. Qian, J. Cavanaugh, I. Khan, et al., ChemPlusChem 81(2016) 718-723; (h) O. Alduhaish, B. Li, H. Arman, et al., Chin. Chem. Lett. 28(2017) 1653-1658. |
| [7] |
(a) J. Rocha, C. D. Brites, L. D. Carlos, Chem. Eur. J. 22(2016) 14782-14795; (b) F. Y. Yi, D. Chen, M. K. Wu, L. Han, H. L. Jiang, ChemPlusChem 81(2016) 675-690; (c) L. Wang, Z. Q. Yao, G. J. Ren, et al., Inorg. Chem. Commun. 65(2016) 9-12; (d) Y. Guo, X. Feng, T. Han, et al., J. Am. Chem. Soc. 136(2014) 15485-15488; (e) Y. Dong, H. Zhang, F. Lei, et al., J. Solid State Chem. 245(2017) 160-163. |
| [8] |
(a) Y. Cui, H. Xu, Y. Yue, et al., J. Am Chem. Soc. 134(2012) 3979-3982; (b) S. N. Zhao, L. J. Li, X. Z. Song, et al., Adv. Funct. Mater. 25(2015) 1463-1469; (c) Y. Yang, L. Chen, F. Jiang, et al., J. Mater. Chem. C 5(2017) 1981-1989. |
| [9] |
(a) D. Zhao, J. Zhang, D. Yue, et al., Chem. Commun. 52(2016) 8259-8262; (b) D. Yue, J. Zhang, D. Zhao, et al., J. Solid State Chem. 241(2016) 99-104. |
| [10] |
(a) R. F. D'Vries, S. Alvarez-Garcia, N. Snejko, et al., J. Mater. Chem. C 1(2013) 6316-6324; (b) X. Fan, S. Freslon, C. Daiguebonne, et al., Inorg. Chem. 54(2015) 5534-5546; (c) Y. Zhou, B. Yan, J. Mater, J. Mater. Chem. C 3(2015) 9353-9358. |
| [11] |
(a) Y. Cui, R. Song, J. Yu, et al., Adv. Mater. 27(2015) 1420-1425; (b) T. Xia, T. Song, Y. Cui, Y. Yang, G. Qian, Dalton Trans. 45(2016) 18689-18695. |
| [12] |
H. Zhang, C. Lin, T. Sheng, et al., Chem. Eur. J. 22 (2016) 4460-4468. DOI:10.1002/chem.201504432 |
| [13] |
D. Zhao, D. Yue, K. Jiang, et al., J. Mater. Chem. C 5 (2017) 1607-1613. DOI:10.1039/C6TC05203G |
| [14] |
(a) A. Cadiau, C. D. Brites, P. Costa, et al., ACS Nano 7(2013) 7213-7218; (b) X. Lian, D. Zhao, Y. Cui, Y. Yang, G. Qian, Chem. Commun. 51(2015) 17676-17679. |
| [15] |
(a) D. Zhao, X. Rao, J. Yu, et al., Inorg. Chem. 54(2015) 11193-11199; (b) X. Rao, T. Song, J. Gao, et al., J. Am. Chem. Soc. 135(2013) 15559-15564. |
| [16] |
(a) S. Katagiri, Y. Hasegawa, Y. Wada, S. Yanagida, Chem. Lett. 33(2004) 1438-1439; (b) K. Miyata, Y. Konno, T. Nakanishi, et al., Angew. Chem. Int. Ed. 52(2013) 6413-6416. |
| [17] |
L. Li, Y. Zhu, X. Zhou, et al., Adv. Funct. Mater. 26 (2016) 8677-8684. DOI:10.1002/adfm.v26.47 |
| [18] |
(a) D. X. Xue, Y. Belmabkhout, O. Shekhah, et al., J. Am. Chem. Soc. 137(2015) 5034-5040; (b) T. Xia, F. Zhu, Y. Cui, et al., J. Solid State Chem. 245(2017) 127-131. |
| [19] |
J. Heine, K. Meller-Buschbaum, Chem. Soc. Rev. 42 (2013) 9232-9242. DOI:10.1039/c3cs60232j |
| [20] |
C. D. S. Brites, A. Millan, L. D. Carlos, Lanthanides in luminescent thermometry, in: J. C. G. Bünzli, V. K. Pecharsky (Eds. ), Handbook on the Physics and Chemistry of Rare Earths, Elsevier Science, B. V., Amsterdam, 2016, pp. 339-427.
|
 2018, Vol. 29
2018, Vol. 29