b School of Materials Science and Engineering, National Institute for Advanced Materials, Nankai University, Tianjin 300350, China;
c Tianjin Key Laboratory of Metal and Molecule-Based Material Chemistry, Key Laboratory of Advanced Energy Materials Chemistry(Ministry of Education), and Collaborative Innovation Center of Chemical Science and Engineering(Tianjin), Nankai University, Tianjin 300071, China
As essential industrial materials, organic dyes are playing key roles in many aspect of relevant human activity and have been widely used in numerous industrial applications such as rubbers, printing, textiles, plastics, and drugs [1]. However, most of dyes are considered be poisonous and stable against oxygen, water, and light, resulting in serious threat to the environment and human health [2-5]. Several approaches, including chemical oxidation, photolytic degradation, biological treatment, and adsorption, have been explored in recent years to treat organic dye wastes [6-8]. Among them, adsorption as a promising method has gained increasing attention because of its efficiency, environment friendly and low-cost [9]. The traditional porous materials, such as activated carbon, zeolites, ion-exchange resins, though have been widely applied in adsorbing dyes [10, 11], the disadvantages of their low adsorption capacities/selectivity and poor modifiability, still inspire the development of high-efficiency absorbent. Therefore, seeking a rapid and effective adsorption approach to remove or recycle dyes from the contaminations is of great imperative.
Metal-organic frameworks (MOFs), due to their tunable pore size, large surface area, modifiable functionality [12-14], have received extensively attentions on their potential applications in gas adsorption/storage [15-18], catalysis [19-21], drug delivery [22, 23], luminescence [24] and so on [25-27]. Recently, many reported MOFs show superior performance than conventional adsorbents in selectively adsorbing organic dyes [28-30]. The ionic MOFs possess incomparable advantages for selective adsorption of cationic or anionic dyes by host-guest electronic interactions [31], which have attracted much research effort. However, among reported ionic MOFs, porous MOFs with cationic framework potentially allowing the capture and separation of bulky anions through anion exchange is very rare [32].
Based on the above consideration, we contemplated an angular ligand, 1, 3-bis(4-carboxyphenyl)imidazolium chloride (H2LCl), which features an imidazolium moiety in the middle and aromatic carboxylate groups at the terminals (Fig. S1 in Supporting information). It is known that the imidazolium salt not only shows good performance as heterogeneous organocatalyst, but also is an ideal linker for the construction of cationic frameworks [33-36]. At the same time, in the current system, we also introduced an N-rich auxiliary ligand, 1H-benzotriazolate (HBTA), which has proved to construct metal cluster secondary building units (SBUs), because the tri-N-donor interior of the triazolate group can provide more coordination sites for metal centers [37]. Motivated by a mixed-ligand strategy, we successfully present a cationic supermolecular MOF based on {Zn4} cluster, {[Zn8(BTA)6(L)5Cl2]·(NO3)3}·5DMF (NUM-4), and there are onedimensional (1D) open channels along a axis. As expected, NUM-4 can rapidly adsorb anionic dyes, including methyl orange (MO), acid orange Ⅱ (AO), Congo red (CR) and methyl blue (MB) based on the anionic exchange, but hardly adsorb neutral Nile red (NR) and cationic methylene blue (MLB), which show that NUM-4 has the ion-selective adsorbing behavior. Furthermore, in practical application, NUM-4 serves as a good adsorbent, which could be easily reused and recycled.
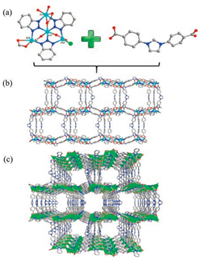
In this work, a coordination compound (NUM-4) was synthesized by the solvothermal reaction of Zn(NO3)2·6H2O, H2L+Cl- and HBTA in DMF solvents upon heating at 85 ℃ for 3 days. The X-ray single-crystal analysis reveals that NUM-4 crystallizes in the monoclinic space group P21/m and exhibits a cationic supermolecular structure based on {Zn4} cluster. The asymmetric unit of NUM-4 comprises four Zn(Ⅱ) centers, two and a half L- ligands, three BTA- and one coordinated Cl-. As shown in Fig. 1, there are four crystallographically independent Zn(Ⅱ) ions (Zn1, Zn2, Zn3 and Zn4), interestingly, all of which adopt different coordinated geometries in each asymmetric unit. Zn1 is 6-coordinated by three carboxylate oxygen atoms from three different L- ligands and three N atoms from three BTA- ligands, and Zn2 is 5-coordinated by three carboxylate oxygen atoms from two L- ligands and two N atoms from two different BTA- ligands. Zn3 also is 6-coordinated by four carboxylate oxygen atoms from three different L- ligands and two N atoms from two BTA- ligands. Zn4 is 4-coordinated by two N atoms from two BTA- ligands and one carboxylate oxygen atom from L- ligand and one Cl- ion. For L-, there are two kinds of coordination modes, one is 4-connected and the other is 6- connected (Fig. S3 in Supporting information).
|
Download:
|
| Fig. 1. (a) The structures of the {Zn4} cluster SBU and L-. (b) The 2D wavelike framework of NUM-4 with porous hexagon windows. (c) The 3D packing structure of NUM-4 showing 1D open channels along a axis (all solvent and hydrogen atoms are omitted for clarity). Color code: Zn, cyan; O, red; N, blue; C gray; Cl green. | |
The coordination modes of Zn(Ⅱ) centers and L- ligand are shown in Fig. 1a. The BTA- ligand uses its three N donors of the triazolate group to "capture" four different Zn(Ⅱ) ions to generate a tetranuclear cluster {Zn4} unit as sub-SBU. Furthermore, these tetranuclear Zinc clusters are linked by carboxylate groups at the terminals of the L- ligands into a 2D wavelike framework with porous hexagon windows ca. 18.8 Å×13.4 Å (Fig. 1b). Actually, two identical sets of 2D layers in NUM-4 are interaction with each other by strongly hydrogen-bonded (C—-H…Cl) between coordinated Cl- and imidazolium moiety of L- to form a 3D supermolecular framework (Fig. S4 in Supporting information). Hence, in the 3D packing arrangement, NUM-4 possesses 1D open channels along a axis (Fig. 1c). PLATON analysis showed that the effective free volume of NUM-4 is 52.8% of the crystal volume after squeezing guest molecules.
The purity and stability of NUM-4 were investigated before further study. As shown in Fig. S5 (Supporting information), the experimental PXRD pattern of the as-synthesized sample is highly consistent with the simulated pattern from the single crystal data, which confirms the phase purity of the bulk sample. Meanwhile, the solvent resistance was studied by immersing the samples in different organic solvents for 24 h, including DMF, DMA, CH2Cl2, CH3OH, and CH3CH2OH, and NUM-4 can still keep its crystalline structure.
Then, we also investigated the thermal stability performance of NUM-4. Thermogravimetric analysis (TGA) curve reveals that the first weight loss of 12% from 25 ℃ to 120 ℃ corresponds to the loss of solvent guests, which is followed by a plateau up to 330 ℃ (Fig. S6 in Supporting information). Further increasing temperature above 330 ℃ results in a steep weight loss, indicative of the decomposition of the frameworks.
To further characterize the pore properties of NUM-4, N2 absorption isotherm for activated NUM-4 (NUM-4a) was collected at 77 K (Fig. S7 in Supporting information), which revealed typical type I behavior with a BET surface area of 976.4 m2/g and Langmuir surface area of 1365.8 m2/g. Subsequently, in order to investigate the gas storage capability of NUM-4a, the low-pressure sorption isotherms of pure-component of CO2 and CH4 were collected at 273 K and 298 K, respectively. As shown in Fig. S8 (Supporting information), the uptake of CO2 reaches 64.3 cm3/g at 273 K under 1 atm and 34.8 cm3/g at 298 K under 1 atm, while the CH4 uptakes are 13.3 cm3/g and 7.6 cm3/g under the corresponding conditions, respectively.
On the basis of the large aperture in the cationic framework of NUM-4, the investigation of dye capture and separation in solution provides more chance to explore the porosity and its potential application. Six different organic dye molecules were chosen to evaluate the absorption ability of this MOF: negatively charged MO, AO Ⅱ, MB and CR, electrically neutral NR, and positively charged MLB (Fig. S9 in Supporting information). Here, 20 mg assynthesized NUM-4 was immersed into six dyes ethanol solutions (20 ppm, 10 mL) at room temperature, respectively. UV–vis spectroscopy of the dye solution was used to record the dye adsorption ability along with the soaking time. The results showed that nearly 95% of the anionic dyes MO, AO, MB and CR could be efficiently absorbed in 720 min. This can be further seen from color change of crystals in which the yellow gradually became orange, orange-red, red and blue towards MO, AO, CR and MB, respectively (Figs. 2a–d). On the contrary, the adsorption of cationic MLB and neutral NR was not observed in 24 h (Figs. 4e–f). The selectivity absorption of anionic dyes by NUM-4 could be attributed to the cationic framework, in which the NO3- anions in the 1D channels of NUM-4 could be exchanged with anionic dyes. It could be concluded that the ion-exchange process for NUM-4 is chargeselective as only the anionic guest could enter and the cationic and neutral ones as denied access. In addition, PXRD patterns confirmed that the crystalline integrity of NUM-4 was retained after the adsorption of the dyes (Fig. S10 in Supporting information).

|
Download:
|
| Fig. 2. UV–vis spectra changes of dyes ethanol solutions (10 mL, 20 ppm) of MO (a), AO (b), CR (c), MB (d), MLB (e), and NR (f) in the presence of NUM-4 (20 mg) monitored with time. The inset photographs are before (left) and after adsorption (right). | |

|
Download:
|
| Fig. 4. UV–vis spectra changes of dye mixture ethanol solution (10 mL, 20 ppm) in the presence of NUM-4 (20 mg): (a) MO&MLB; (b) MO&NR. The inset photographs are before (left) and after adsorption (right) | |
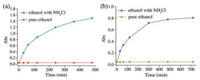
To further confirm that the selective dye adsorption is an ionexchange process, dye release from MO@ NUM-4 and AO@ NUM-4 was investigated. Crystals of NUM-4 were first soaked in ethanol solution of MO and AO for 24 h to make them saturated. 20 mg of the saturated crystals were added to 10 mL pure ethanol and a saturated ethanol solution of NH4Cl, respectively. UV–vis spectra were used to record the release ability of NUM-4. As shown in Fig. 3, the dye molecules in MO@NUM-4 and AO@NUM-4 could be released quickly in the presence of NH4Cl as the trigger. The release reached a plateau within 500 min. On the other hand, the intensity of MO and AO in ethanol solution gradually increases along with the time (Figs. S11 and S12 in Supporting information). However, they are difficult to release in pure ethanol. This result further proves that the process of MO and AO release is the fact of anionexchange as proposed above. In addition, the PXRD of releasedMO@NUM-4 and released-AO@NUM-4 showed the same patterns as NUM-4, indicating the stability of the crystalline sample (Fig. S10 in Supporting information).
|
Download:
|
| Fig. 3. The release rate comparison of MO from MO@NUM-4 (a) and AO from AO@NUM-4 (b) in pure ethanol and an NH4Cl saturated solution of ethanol, respectively | |
In the end, the adsorption selectivity of NUM-4 was also tested by soaking the freshly ethanol-exchanged samples (20 mg) in ethanol solution of a mixture of MO & MLB or MO & NR (20 ppm, 10 mL) at room temperature, respectively. The concentration of dye in the solution was recorded by UV–vis absorption spectroscopy. Evidently, as indicated in Fig. 4, the concentrations of MO in the solution drastically decreased while the concentrations of MLB and NR were nearly constant with time. The results revealed that NUM- 4 can rapidly adsorb anionic dye molecules, while cationic and neutral dye molecules were left in the solution. Interestingly, the colors for two groups of dye mixtures changed obviously, from green to blue for MO/MLB mixture, and from red to pink for MO/NR mixture, respectively. From the above experiments, we can further infer that NUM-4 might be a good adsorbent for efficient and selective removal of anionic dyes from effluents.
In summary, a unique cluster-based supermolecular framework, NUM-4, was successfully constructed with a cationic imidazolium-based dicarboxylate ligand and an N-rich auxiliary ligand. Due to the cationic network and guest anionic molecules in 1D channels, NUM-4 can rapidly and selectively adsorb anionic dyes (MO, AO, CR and MB) in ethanol solution and release MO and AO easily based on the charge-exclusive effect. Dye adsorption and release studies revealed that NUM-4 could be the promising materials to efficiently and selectively remove anionic dye molecules from effluents, which is very significant for environmental cleanup.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21371102, 21531005 and 21673120), and the Natural Science Foundation of Tianjin (Nos. 16JCZDJC36900 and 15JCZDJC38800).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.10.003.
| [1] |
B.O. Okesola, D.K. Smith, Chem. Soc. Rev. 45 (2016) 4226-4251. DOI:10.1039/C6CS00124F |
| [2] |
Y.Y. Jia, G.J. Ren, A.L. Li, et al., Cryst. Growth Des. 16 (2016) 5593-5597. DOI:10.1021/acs.cgd.6b00813 |
| [3] |
Y. Han, S. Sheng, F. Yang, et al., J. Mater. Chem. A 3 (2015) 12804-12809. DOI:10.1039/C5TA00963D |
| [4] |
Y.X. Tan, Y.P. He, M. Wang, J. Zhang, RSC Adv. 4 (2014) 1480-1483. DOI:10.1039/C3RA41627E |
| [5] |
S. Yao, T. Xu, N. Zhao, et al., Dalton Trans. 46 (2017) 3332-3337. DOI:10.1039/C7DT00192D |
| [6] |
Y.C. He, J. Yang, W.Q. Kan, et al., J. Mater. Chem. A 3 (2015) 1675-1681. DOI:10.1039/C4TA05391E |
| [7] |
B. Manna, S. Singh, A. Karmakar, A.V. Desai, S.K. Ghosh, Inorg. Chem. 54 (2014) 110-116. |
| [8] |
C.C. Wang, J.R. Li, X.L. Lv, Y.Q. Zhang, G. Guo, Energy Environ. Sci. 7 (2014) 2831-2867. DOI:10.1039/C4EE01299B |
| [9] |
Y. Wong, Y. Szeto, W. Cheung, G. McKay, Langmuir 19 (2003) 7888-7894. DOI:10.1021/la030064y |
| [10] |
D.D. Hu, J. Lin, Q. Zhang, et al., Chem. Mater. 27 (2015) 4099-4104. DOI:10.1021/acs.chemmater.5b01158 |
| [11] |
Y.C. He, J. Yang, W.Q. Kan, J.F. Ma, CrystEngComm 15 (2013) 848-851. DOI:10.1039/C2CE26836A |
| [12] |
Q. Gao, J. Xu, D. Cao, Z. Chang, X.H. Bu, Angew. Chem. Int. Ed. 55 (2016) 15027-15030. DOI:10.1002/anie.201608250 |
| [13] |
X. Zhao, X. Bu, E.T. Nguyen, et al., J. Am. Chem. Soc. 138 (2016) 15102-15105. DOI:10.1021/jacs.6b07901 |
| [14] |
Y.Y. Jia, Y.H. Zhang, J. Xu, et al., Chem. Commun. 51 (2015) 17439-17442. DOI:10.1039/C5CC07249B |
| [15] |
X.T. Liu, Y.Y. Jia, Y.H. Zhang, et al., Inorg. Chem. Front. 3 (2016) 1510-1515. DOI:10.1039/C6QI00191B |
| [16] |
T.L. Hu, H. Wang, B. Li, et al., Nat. Commun. 6 (2015) 7328-7336. DOI:10.1038/ncomms8328 |
| [17] |
O. Alduhaish, B. Li, H. Arman, et al., Chin. Chem. Lett. 28 (2017) 1653-1658. DOI:10.1016/j.cclet.2017.04.025 |
| [18] |
L. Liu, S.G. Telfer, J. Am. Chem. Soc. 137 (2015) 3901-3909. DOI:10.1021/jacs.5b00365 |
| [19] |
F.R. Fortea Perez, M. Mon, J. Ferrando Soria, et al., Nat. Mater. 16 (2017) 760-766. |
| [20] |
Q. Yang, Q. Xu, H.L. Jiang, Chem. Soc. Rev. 46 (2017) 4774-4808. DOI:10.1039/C6CS00724D |
| [21] |
X.L. Ni, J. Liu, Y.Y. Liu, et al., Chin. Chem. Lett. 28 (2017) 1057-1061. DOI:10.1016/j.cclet.2017.01.020 |
| [22] |
H. Wang, T.L. Hu, R.M. Wen, Q. Wang, X.H. Bu, J. Mater. Chem. B 1 (2013) 3879-3882. DOI:10.1039/c3tb20633e |
| [23] |
M.X. Wu, Y.W. Yang, Adv. Mater. 29 (2017) 1606134-1606153. DOI:10.1002/adma.201606134 |
| [24] |
L. Chen, J.W. Ye, H.P. Wang, et al., Nat. Commun. 8 (2017) 15985-15994. DOI:10.1038/ncomms15985 |
| [25] |
Y. Chen, S. Zhang, S. Cao, et al., Adv. Mater. 29 (2017) 1606221-1606226. DOI:10.1002/adma.201606221 |
| [26] |
Y.Z. Chen, Z.U. Wang, H. Wang, et al., J. Am. Chem. Soc. 139 (2017) 2035-2044. DOI:10.1021/jacs.6b12074 |
| [27] |
S.P. Guo, G.C. Guo, J. Mater. Chem. A 2 (2014) 20621-20628. DOI:10.1039/C4TA04757E |
| [28] |
X. Zhang, Y. Gao, H. Liu, Z. Liu, CrystEngComm 17 (2015) 6037-6043. DOI:10.1039/C5CE00862J |
| [29] |
J.A. Johnson, X. Zhang, T.C. Reeson, Y.S. Chen, J. Zhang, J. Am. Chem. Soc. 136 (2014) 15881-15884. DOI:10.1021/ja5092672 |
| [30] |
X. Zhao, X. Bu, T. Wu, et al., Nat. Commun. 4 (2013) 2344-2352. DOI:10.1038/ncomms3344 |
| [31] |
Z. Zhu, Y.L. Bai, L. Zhang, et al., Chem. Commun. 50 (2014) 14674-14677. DOI:10.1039/C4CC07365G |
| [32] |
H. Fei, D.L. Rogow, S.R. Oliver, et al., J. Am. Chem. Soc. 132 (2010) 7202-7209. DOI:10.1021/ja102134c |
| [33] |
C.I. Ezugwu, B. Mousavi, M.A. Asrafa, et al., Catal. Sci. Technol. 6 (2016) 2050-2054. DOI:10.1039/C5CY01944C |
| [34] |
G. Nickerl, A. Notzon, M. Heitbaum, et al., Cryst. Growth Des. 13 (2013) 198-203. DOI:10.1021/cg301347t |
| [35] |
S. Sen, N.N. Nair, T. Yamada, H. Kitagawa, P.K. Bharadwaj, J. Am. Chem. Soc. 134 (2012) 19432-19437. DOI:10.1021/ja3076378 |
| [36] |
L. Wang, W.W. He, Z.Q. Yao, T.L. Hu, ChemistrySelect 2 (2017) 283-287. DOI:10.1002/slct.201601666 |
| [37] |
Y.W. Li, J.R. Li, L.F. Wang, et al., J. Mater. Chem. A 1 (2013) 495-499. DOI:10.1039/C2TA00635A |
 2018, Vol. 29
2018, Vol. 29 


