b College of Science, China University of Petroleum(East China), Qingdao 266580, China
Metal-organic frameworks (MOFs), an emerging class of multifunctional porous materials, have attracted extensive attentions due to their unprecedented surface areas, variable structures and tunable functionalities [1-4]. Thanks to these characteristics, they have found plenty of applications including gas storage [5, 6] and separation [7, 8], catalysis [9-11], drug delivery [12], photoelectronics [13] and sensing [14] etc. Furthermore, the derivatives of MOFs also have displayed promising potentials in energy storage and energy conversion technologies [15-19]. The prerequisite for realizing real-world applications is the ability to produce MOFs at large scale, and at the same time ensuring high quality and low cost of the product. However, the efficient synthetic technique combining with continuous production, high productivity and good product quality remains a critical issue for practical applications [20]. In order to diminish the gaps between laboratory synthesis and industrial production, some synthetic techniques, such as sonchemical [21, 22], microwave [23, 24], spray drying [25], electrochemical [26], aerosol [27], mechanochemical [28, 29], and flow chemistry synthesis [30-32], have been explored. Despite of significant advances, few MOFs appear to be commercially available at large quantities (kilogram scale) [33, 34]. Therefore, the development of facial and efficient synthetic methods for MOFs is essential.
Zr-based MOFs, typically contain multi-topic Zr-O clusters connected by organic ligands, exhibit high porosity, excellent thermal/hydro stability, rich topologies and tunable functionalities [35, 36]. Owing to these merits, they have been considered as one of the most promising candidates among MOFs for practical applications [37]. Although several Zr-based MOFs have been reported in recent years [38-40], the investigation of synthetic methodologies for Zr-based MOFs is still rare. Recently, some groups have demonstrated that the UiO-66 can be synthesized through flow reaction [41-44]. Nevertheless, such works are still far from adequate for practical applications, and the general synthetic strategy that is applicable for Zirconium MOFs (Zr-MOFs) comprised of different structures and topologies is highly desired. In this work, we report a method of continuous production for Zrbased MOFs through a microdroplet flow reaction strategy. Four types of Zr-MOFs, namely MOF-801 [45], MOF-804 [46], DUT-67 [47] and MOF-808 [48], which bearing different secondary building units (SBUs) and topologies, were prepared in high yield, high quality and high productivity (termed as MF-MOF-801, MFMOF-804, MF-DUT-67, and MF-MOF-808, respectively, where the MF represents "microdroplet flow"). Structural studies revealed the nearly same properties of these MOFs compared to that derived from conventional solvothermal synthesis (termed as ST-MOF-801, ST-MOF-804, ST-DUT-67, and ST-MOF-808, respectively, where the ST represents "solvothermal"). Gas-sorption studies of these Zrbased MOFs revealed outstanding CO2 uptake at low pressures and high CO2/N2 selectivity.
MOF-801 is a Zr-MOF formed by the linkage of fumaric acid and Zr6(μ-O)4(OH)4(COO)12 SBU [45]. The short ligand length of fumaric acid gives rise to a narrow pore window (0.48 nm), which is the smallest among Zr-series MOFs. MOF-804 is a fcu type MOF assembled from the Zr6(μ-O)4(OH)4(COO)12 SBU and 2, 5-dihydroxyterephthalic acid, which bearing phenolic hydroxyl groups on ligand [46]. DUT-67 is a Zr-MOF composed of the Zr6(μ-O)4(OH)4(COO)8 SBU and thiophene-2, 5-dicarboxylate acid (H2TDC) [47]. The V-shaped ligand leads to a reo network, a rare topology in Zr-MOFs. MOF-808 is a spn type Zr-MOF assembled from H3BTC and Zr6(μ-O)4(OH)4(COO)6 SBU, in which a meso-scale cage is involved in the framework [48]. Owing to the distinctive steric extension and functional groups in ligands and different framework topologies of resultant MOFs, these Zr-MOFs were selected as research objects in this work. They were synthesized through a home-built microdroplet flow system which comprises of three syringe pumps connected by one micro-mixer, one heating coil and sample collecting unit (Scheme 1). In a typical procedure, both solutions of metal salt and organic ligand were pumped into the static micro-mixer equipped with a magnetic stirring to form a homogeneous solution. Organic acid (formic acid and acetic acid) or inorganic acid (HCl) was added into above solution as the modulator. Then, the solution of reactants and oil phase was pumped into a micro-pipeline co-currently at a controlled flow rate to form uniform and dispersed microdroplets. These droplets passed through a heating coil (2 mm i.d. and 3 mm o.d., and the length is 2 m) that were immersed in a heating bath. White precipitate formed in these microdroplets. Afterwards, these microdroplets were cooled down when passing through a cooling coil immersed in ice-water bath. The precipitate was separated from mixed phase (including the oil, organic solvent and samples) by centrifuging at 5000 rpm for 10 min and washed with Soxhlet extraction using petroleum ether and ethanol as the eluent. These samples were soaked in the ethanol before characterization.
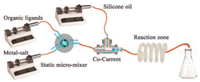
|
Download:
|
| Scheme 1. Schematic representation of the general microdroplet flow reaction. | |
Powder X-ray diffraction (PXRD) patterns confirm that all of these MOFs prepared under MF conditions possess the identical structure with crystallographic data of MOF-801, MOF-804, DUT- 67 and MOF-808, respectively (Figs. 1a–d) [45-48]. No extra peaks were observed on PXRD patterns, demonstrating high purity of these samples. The high degree of crystallinity is revealed by the sharp peaks in PXRD patterns. Notably, the crystallinity of these MOFs is even higher than that derived from conventional solvothermal synthesis, especially for MOF-808, which exhibited a weak crystallinity when prepared under solvothermal condition (see PXRD patterns in Figs. S1–S4 in Supporting information). These results suggest that the microdroplet flow reaction can produce pure and highly crystalline Zr-MOFs. The quality of the obtained Zr-MOFs can also be characterized by scanning electron microscopy (SEM). As shown in Fig. 1a, a pure phase of regular polyhedral crystals is observed for MF-MOF-801. The crystals of MF-MOF-801 exhibit a uniform dimension of about 300 nm. Different from MF-MOF-801, the MOF-801 derived from conventional solvothermal synthesis (ST-MOF-801) exhibits an inhomogeneous phase of particles with irregular shapes and non-uniform grain sizes (in the range of 2–5 μm, Fig. S5 in Supporting information). MF-MOF-804 exhibits a phase of regular polyhedral crystals. The crystal sizes are in the range of 100–500 nm (Fig. 1b and Fig. S6 in Supporting information). As shown in Fig. 1c, MFDUT-67 exhibits a phase of crystals with regular polyhedron crystals (3–5 μm), which is coexisted with some nano-scale crystal grains (200–500 nm). The morphology and crystal size of MF-DUT- 67 is similar to that of ST-DUT-67 (Fig. S7 in Supporting information). MF-MOF-808 exhibits a single phase of regular hexahedral crystals with a dimension of about 300 nm. The regular shapes and sizes of crystals for MF-MOF-808 show a clear contrast with that derived from conventional solvothermal synthesis, under which the obtained ST-MOF-808 exhibit irregular shapes and sizes (Fig. 1d and Fig. S8 in Supporting information).

|
Download:
|
| Fig. 1. PXRD patterns and SEM images of the crystal obtained by microdroplet flow synthesis: (a) MF-MOF-801, (b) MF-MOF-804, (c) MF-DUT-67 and (d) MF-MOF-808. | |
Although these MOFs were synthesized in a short period (32– 80 min) under MF conditions, all of products possess high purity and crystallinity. In contrast, the solvothermal synthesized MOFs exhibit a much weaker crystallinity and product quality, even though a long reaction time (e.g., 24 h) is employed under solvothermal conditions. Above observations hints the superiority of MF synthesis to conventional solvothermal synthesis in terms of product quality. The much smaller sizes combined with uniform morphologies can render these MOFs with broader application prospects. The high quality of crystals can be attributed to the unique advantages of microdroplet flow reaction synthesis. The large surface-area-to-volume ratio, the efficient exchange of mass/heat and the rapidly flow recirculation of reactants in microdroplet flow reaction conditions can accelerate crystal nucleation and advance crystal growth [49-52]. With accelerated nucleation and crystal growth process, high quality Zr-MOFs can be formed in a short reaction time under MF conditions.
Beside high quality, the production rate is also an essential aspect for MOF production as it is highly correlated with cost. The yield calculated from the actual weight of activated MOFs divided by theoretical weight is shown in Fig. 2a. In solvothermal synthesis, the yield for ST-MOF-801 is 65.7% (calcd. on the basis of fumaric acid) after 24 h of solvothermal reaction, which is consistent with the literature results [48]. In comparison, a yield of 85.2% is obtained for MF-MOF-801 after 32 min of reaction under MF conditions, 30% higher than that derived from solvothermal synthesis. The much higher yield is also found on other Zr-MOFs. The higher yield could be attributed to the functions of MF conditions, under which the more efficient mass/heat transfer lead to the higher efficient conversion of reactants. With higher conversion rate in a short reaction time, very high STY [41] was observed for these MOFs. As shown in Fig. 2b, the STY value for MFMOF-801 was 367.2 kg m-3 d-1, which is 29.3 times higher than that of ST-MOF-801 (12.5 kg m-3 d-1). The STY values for MF-MOF- 804, MF-DUT-67 and MF-MOF-808 were calculated as 247.1, 114.3 and 136.4 kg m-3 d-1, respectively, which were also much higher than STY values of corresponding MOFs derived from solvothermal synthesis (Table S1 in Supporting information). The remarkable differences highlight the superiority of MF reaction to conventional solvothermal synthesis in terms of productivity, that MF reaction can produce much higher amount of MOFs at a given time and space. The excellent productivity renders this method to be very promising for practical application.

|
Download:
|
| Fig. 2. (a) High conversion and (b) space-time yield (STY) of these crystals obtained by microdroplet flow synthesis and conventional solvothermal synthesis of MFMOF-801, MF-MOF-804, MF-DUT-67 and MF-MOF-808. | |
N2 isotherms at 77 K were measured on the activated samples of these Zr-MOFs to evaluate their pore structures. As shown in Fig. 3, all of these samples exhibited a Type-I isotherm with a steep increase in the low pressure range and a plateau in the middle pressure range, suggesting an overall microporous nature. The Brunauer–Emmett–Teller (BET) surface areas of MF-MOF-801, MFMOF-804, MF-DUT-67 and MF-MOF-808 were calculated as 833.4, 481.8, 679.1 and 1842.2 m2/g, respectively. Pore size distributions (PSD) calculated from nonlocal density function theory (NLDFT) demonstrated the microporous structure. MF-MOF-801, MF-MOF- 804, MF-DUT-67 and MF-MOF-808 possessed total pore volume values of 0.413, 0.703, 0.314 and 1.263 cm3/g, respectively (Table S2 in Supporting information). The BET surface areas for these MOFs are comparable to those derived from solvothermal synthesis, except of MF-MOF-801, which showed a higher BET surface area than ST-MOF-801. This higher BET surface area can be attributed to the higher quality of obtained MOFs. Thermal gravimetric analysis (TGA) demonstrated that all of these MOFs exhibited excellent thermal stability in the air. These as-synthesized samples of MFMOF-801, MF-MOF-804, MF-DUT-67 and MF-MOF-808 show the thermal stability up to 363 ℃, 294 ℃, 398 ℃ and 529 ℃ respectively, which are almost identical with the MOFs prepared from conventional solvothermal methods (Figs. S9–S12 in Supporting information).

|
Download:
|
| Fig. 3. N2 sorption isotherms at 77 K and pore size distribution obtained from Nonlocal density function theory (NLDFT) on (a) MF- and ST-MOF-801; (b) MF- and ST-MOF- 804; (c) MF- and ST-DUT-67; (d) MF- and ST-MOF-808. | |
The different porous structures for these Zr-based MOFs encourage us to investigate their CO2 adsorption properties which are relevant with CO2 capture from flue gas. As shown in Fig. 4a, CO2 adsorption isotherms were measured at 298 K on the activated samples of these Zr-based MOFs in the pressure range of 0–1 bar. CO2 uptake at 298 K and 1 bar on MF-MOF-801, MF-MOF-804, MFDUT-67 and MF-MOF-808 are 1.37, 1.00, 1.98 and 2.00 mmol/g, respectively. Comparatively, rather small amount of N2 was adsorbed on these MOFs, which can be ascribed to the zero quadrupole moments for N2 molecules. The higher adsorption affinity for CO2 compared with the limited uptake of N2 indicated the significant improvement in the CO2/N2 adsorptive selectivity of these Zr-based MOFs. To predict CO2/N2 binary mixture selectivity, the ideal adsorption solution theory (IAST) calculation, whose reliability has been verified on several MOFs and other adsorbents [53-55], was used to evaluate the gas adsorption selectivity of CO2/N2. CO2/N2 Selectivities of MF-MOF-801, MFMOF-804, MF-DUT-67 and MF-MOF-808 for equal fractions of gas mixtures (CO2/N2 = 50/50, v/v) in the pressure range of 0–1 bar were revealed in Fig. 4b. The CO2/N2 selectivity on these Zr-MOFs are 31.1, 57.2, 26.9 and 9.14 at 298 K and 1 bar, respectively. The MFMOF-804 exhibits the highest CO2/N2 selectivity among those MOFs and the superior CO2/N2 selectivity can be ascribed to the function of decorated phenolic hydroxyl groups [56]. The polar phenolic hydroxyl groups on MOF-804 can increase the hydrophilic of frameworks, thus increasing the interaction strength of CO2 with the frameworks.[The enhanced interaction strength is verified by the high Qst (~33 kJ/mol, Figs. S13–S16 in Supporting information). Such observations are consistent with the recent reports in CO2 adsorption [57, 58]. The outstanding CO2/N2 selectivity combined with excellent stability allows MOF-804 to be a promising candidate for CO2 capture.

|
Download:
|
| Fig. 4. (a) CO2 and N2 adsorption isotherms on MF-MOF-801, MF-MOF-804, MF-DUT-67 and MF-MOF-808 at 298 K and 0–1 bar; (b) The predicted CO2/N2 selectivity of MFMOF-801, MF-MOF-804, MF-DUT-67 and MF-MOF-808 at different pressures. | |
In summary, a series of Zr-based MOFs: MOF-801, MOF-804, DUT-67 and MOF-808, which comprise of different ligands and topologies, were continuously synthesized via a microdroplet flow reaction. Compared to MOFs derived from conventional solvothermal synthesis, the MOFs obtained from microdroplet flow reaction possess a higher degree of crystallinity and more uniform morphologies. Besides, these Zr-MOFs exhibit higher yields and much superior space-time yields compared to that prepared from solvothermal synthesis, even though microdroplet flow reaction proceeds with a short reaction time. The space-time yield values for MF-MOF-801, MF-MOF-804, MF-DUT-67 and MF-MOF-808 are highly up to 367.2, 247.1, 114.3, and 136.4 kg m-3 d-1, respectively, which outperforms current synthetic technologies. N2 isotherms and TGA plots of these Zr-MOFs that prepared from microdroplet flow reaction demonstrate the similar pore structure and thermal stability with that derived from solvothermal synthesis. Gas sorption studies reveal that the MOF-804, which is decorated with phenolic hydroxyl groups on ligand, exhibits the higher CO2/N2 selectivity over other Zr-MOFs. The continuous synthesis, good product quality and high productivity of microdroplet flow reaction are expected to realize large-scale production for ZrMOFs in practical applications.
AcknowledgmentsThis work was supported by grants from the National Natural Science Foundation of China (Nos. 21401215, 21473254) and the Special Project Fund of "Taishan Scholars" of Shandong Province (No. ts201511017).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.09.057.
| [1] |
S.S.Y. Chui, S.M.F. Lo, J.P.H. Charmant, et al., Science 283 (1999) 1148-1150. DOI:10.1126/science.283.5405.1148 |
| [2] |
H. Li, M. Eddaoudi, M. O'Keeffe, O.M. Yaghi, Nature 402 (1999) 276-279. DOI:10.1038/46248 |
| [3] |
M. Eddaoudi, J. Kim, N. Rosi, et al., Science 295 (2002) 469-472. DOI:10.1126/science.1067208 |
| [4] |
H.C. Zhou, S. Kitagawa, Chem. Soc. Rev. 43 (2014) 5415-5418. DOI:10.1039/C4CS90059F |
| [5] |
X. Zhao, B. Xiao, A.J. Fletcher, et al., Science 306 (2004) 1012-1015. DOI:10.1126/science.1101982 |
| [6] |
L. Li, J.G. Bell, S. Tang, et al., Chem. Mater. 26 (2014) 4679-4695. DOI:10.1021/cm403697m |
| [7] |
J.H. Cavka, C.A. Grande, G. Mondino, R. Blom, Ind. Eng. Chem. Res. 53 (2014) 15500-15507. DOI:10.1021/ie500421h |
| [8] |
O. Alduhaish, B. Li, H. Arman, et al., Chin. Chem. Lett. 28 (2017) 1653-1658. DOI:10.1016/j.cclet.2017.04.025 |
| [9] |
Q. Yang, Q. Xu, H.L. Jiang, Chem. Soc. Rev. 46 (2017) 4774-4808. DOI:10.1039/C6CS00724D |
| [10] |
M. Rimoldi, A.J. Howarth, M.R. DeStefano, et al., ACS Catal. 7 (2017) 997-1014. DOI:10.1021/acscatal.6b02923 |
| [11] |
Z. Hu, Y. Peng, Y. Gao, et al., Chem. Mater. 28 (2016) 2659-2667. DOI:10.1021/acs.chemmater.6b00139 |
| [12] |
K.M. Taylor-Pashow, J. Della Rocca, Z. Xie, et al., J. Am. Chem. Soc. 131 (2009) 14261-14263. DOI:10.1021/ja906198y |
| [13] |
B. Dhara, P.P. Patra, P.K. Jha, et al., J. Phys. Chem. C 118 (2014) 19287-19293. DOI:10.1021/jp503875w |
| [14] |
A. Douvali, A.C. Tsipis, S.V. Eliseeva, et al., Angew. Chem. Int. Ed. 54 (2015) 1651-1656. DOI:10.1002/anie.201410612 |
| [15] |
L. Yan, L. Cao, P. Dai, et al., Adv. Funct. Mater. 27 (2017) 1703455. DOI:10.1002/adfm.v27.40 |
| [16] |
L. Li, P. Dai, X. Gu, et al., J. Mater. Chem. A 5 (2017) 789-795. DOI:10.1039/C6TA08016B |
| [17] |
L. Yan, P. Dai, Y. Wang, et al., ACS Appl. Mater. Interfaces 9 (2017) 11642-11650. DOI:10.1021/acsami.7b01037 |
| [18] |
J.W. Ding, R. Wang, Chin. Chem. Lett. 27 (2016) 655-658. DOI:10.1016/j.cclet.2016.03.005 |
| [19] |
Y.Y. Zheng, C.X. Li, X.T. Ding, et al., Chin. Chem. Lett. 28 (2017) 1473-1478. DOI:10.1016/j.cclet.2017.03.014 |
| [20] |
B. Seoane, S. Castellanos, A. Dikhtiarenko, et al., Coord. Chem. Rev. 307 (2015) 147-187. |
| [21] |
V. Safarifard, A. Morsali, Coord. Chem. Rev. 292 (2015) 1-14. DOI:10.1016/j.ccr.2015.02.014 |
| [22] |
L.G. Qiu, Z.Q. Li, Y. Wu, et al., Chem. Commun. (2008), 3642-3644. |
| [23] |
Z. Ni, R.I. Masel, J. Am. Chem. Soc. 128 (2006) 12394-12395. DOI:10.1021/ja0635231 |
| [24] |
H. Bux, F. Liang, Y. Li, et al., J. Am. Chem. Soc. 131 (2009) 16000-16001. DOI:10.1021/ja907359t |
| [25] |
A. Carne-Sanchez, I. Imaz, M. Cano-Sarabia, D. Maspoch, Nat. Chem. 5 (2013) 203-211. DOI:10.1038/nchem.1569 |
| [26] |
A. Martinez Joaristi, J. Juan-Alcañiz, P. Serra-Crespo, et al., Cryst. Growth Des. 12 (2012) 3489-3498. DOI:10.1021/cg300552w |
| [27] |
A. Garcia Marquez, P. Horcajada, D. Grosso, et al., Chem. Commun. 49 (2013) 3848-3850. DOI:10.1039/c3cc39191d |
| [28] |
O. Shekhah, Y. Belmabkhout, K. Adil, et al., Chem. Commun. 51 (2015) 13595-13598. DOI:10.1039/C5CC04487A |
| [29] |
D. Crawford, J. Casaban, R. Haydon, et al., Chem. Sci. 6 (2015) 1645-1649. DOI:10.1039/C4SC03217A |
| [30] |
M. Rubio-Martinez, C. Avci-Camur, A.W. Thornton, et al., Chem. Soc. Rev. 46 (2017) 3453-3480. DOI:10.1039/C7CS00109F |
| [31] |
L. Paseta, B. Seoane, D. Julve, et al., ACS Appl. Mater. Interfaces 5 (2013) 9405-9410. DOI:10.1021/am4029872 |
| [32] |
Y. Peng, W.K. Wong, Z. Hu, et al., Chem. Mater. 28 (2016) 5095-5101. DOI:10.1021/acs.chemmater.6b01954 |
| [33] |
U. Mueller, M. Schubert, F. Teich, et al., J. Mater. Chem. 16 (2006) 626-636. DOI:10.1039/B511962F |
| [34] |
J.H. Cavka, S. Jakobsen, U. Olsbye, et al., J. Am. Chem. Soc. 130 (2008) 13850-13851. DOI:10.1021/ja8057953 |
| [35] |
C. Wang, X. Liu, N. Keser Demir, et al., Chem. Soc. Rev. 45 (2016) 5107-5134. DOI:10.1039/C6CS00362A |
| [36] |
D. Feng, Z.Y. Gu, Y.P. Chen, et al., J. Am. Chem. Soc. 136 (2014) 17714-17717. DOI:10.1021/ja510525s |
| [37] |
F. Vermoortele, R. Ameloot, A. Vimont, et al., Chem. Commun. 47 (2011) 1521-1523. DOI:10.1039/C0CC03038D |
| [38] |
I. Stassen, M. Styles, T. Van Assche, et al., Chem. Mater. 27 (2015) 1801-1807. DOI:10.1021/cm504806p |
| [39] |
W. Liang, D.M. D'Alessandro, Chem. Commun. 49 (2013) 3706-3708. DOI:10.1039/c3cc40368h |
| [40] |
K. Uzarevic, T.C. Wang, S.Y. Moon, et al., Chem. Commun. 52 (2016) 2133-2136. DOI:10.1039/C5CC08972G |
| [41] |
M. Faustini, J. Kim, G.Y. Jeong, et al., J. Am. Chem. Soc. 135 (2013) 14619-14626. DOI:10.1021/ja4039642 |
| [42] |
M. Rubio-Martinez, M.P. Batten, A. Polyzos, et al., Sci. Rep. 4 (2014) 5443. |
| [43] |
M. Taddei, D.A. Steitz, J.A. van Bokhoven, M. Ranocchiari, Chem. Eur. J. 22 (2016) 3245-3249. DOI:10.1002/chem.201505139 |
| [44] |
S. Tai, W. Zhang, J. Zhang, et al., Microporous Mesoporous Mater. 220 (2016) 148-154. DOI:10.1016/j.micromeso.2015.08.037 |
| [45] |
G. Wißmann, A. Schaate, S. Lilienthal, et al., Microporous Mesoporous Mater. 152 (2012) 64-70. DOI:10.1016/j.micromeso.2011.12.010 |
| [46] |
D. Cunha, C. Gaudin, I. Colinet, et al., J. Mater. Chem. B 1 (2013) 1101-1108. DOI:10.1039/c2tb00366j |
| [47] |
V. Bon, I. Senkovska, I.A. Baburin, S. Kaskel, Cryst. Growth Des. 13 (2013) 1231-1237. DOI:10.1021/cg301691d |
| [48] |
H. Furukawa, F. Gandara, Y.B. Zhang, et al., J. Am. Chem. Soc. 136 (2014) 4369-4381. DOI:10.1021/ja500330a |
| [49] |
N. Hassan, A. Stocco, A. Abou-Hassan, J. Phys. Chem. C 119 (2015) 10758-10765. DOI:10.1021/acs.jpcc.5b02527 |
| [50] |
H. Song, J.D. Tice, R.F. Ismagilov, Angew. Chem. Int. Ed. 42 (2003) 768-772. DOI:10.1002/anie.200390203 |
| [51] |
H. Song, D.L. Chen, R.F. Ismagilov, Angew. Chem. Int. Ed. 45 (2006) 7336-7356. DOI:10.1002/(ISSN)1521-3773 |
| [52] |
S. Duraiswamy, S.A. Khan, Small 5 (2009) 2828-2834. DOI:10.1002/smll.v5:24 |
| [53] |
Y.S. Bae, K.L. Mulfort, H. Frost, et al., Langmuir 24 (2008) 8592-8598. DOI:10.1021/la800555x |
| [54] |
T. Pham, K.A. Forrest, P. Nugent, et al., J. Phys. Chem. C 117 (2013) 9340-9354. DOI:10.1021/jp402304a |
| [55] |
Y.L. Xu, Q. Gao, M. Zhao, et al., Chin. Chem. Lett. 28 (2017) 55-59. DOI:10.1016/j.cclet.2016.06.006 |
| [56] |
H.L. Jiang, D. Feng, T.F. Liu, et al., J. Am. Chem. Soc. 134 (2012) 14690-14693. DOI:10.1021/ja3063919 |
| [57] |
Y. Bai, Y. Dou, L.H. Xie, et al., Chem. Soc. Rev. 45 (2016) 2327-2367. DOI:10.1039/C5CS00837A |
| [58] |
B. Seoane, J. Coronas, I. Gascon, et al., Chem. Soc. Rev. 44 (2015) 2421-2454. DOI:10.1039/C4CS00437J |
 2018, Vol. 29
2018, Vol. 29 


