b State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China
Over the past two decades, metal-organic frameworks (MOFs), emerging as a new class of porous crystalline materials, developed at an uncommon pace [1-3]. They extended the field of porous materials and presented excellent potential in various applications, including catalysts, chemical sensing, gas separation and drug delivery and so on [4-9]. Among these properties, the application of MOFs in heterogeneous catalysis has attracted increasing attentions recently. It is well known that many advantages are associated with heterogeneous catalysis, including high activity and selectivity, as well as the convenience for separation of the surfactants from the reaction systems [10]. Previous endeavours devoted to take advantage of its high surface area and prepare composite heterogeneous catalysts by embedding nanoparticles into its pore structure. Up to now, there have been many successful examples concerning NPs@MOFs and its catalytic activities. Particularly, Pt@UIO-66, Pd@UIO-66, Au@ZIF-8, Pd@ZIF-8, Pt@MIL-101, Ag@BIF-51, Ag@Ln-MOFs, Ag@Zn-MOF and similar results have been reported [11, 12]. Given that composite materials based on MOFs have been widely used as catalysts, it is logic to consider whether these MOFs whose metal sites are noble metals (such as Ag(Ⅰ)) can be employed as solid catalysts directly. Compared to traditional catalytic materials, MOFs have accurate structure and component information, which help to reveal the mechanism during the catalytic process. Meanwhile, MOFs can be chemically modified via post-synthetic approach, consequently, endowing MOFs with new opportunities [13].
Actually, several silver coordination compounds have been proved to be efficient catalysts for many organic reactions, such as photocatalytic degradation of phenol, cycloaddition of azides and alkynes, oxygenation of sulfide and partial oxidation of primary amines [14]. The use of some Ag-MOFs as biocide material in water treatment and biomedical applications also has been evaluated [15]. Apart from above reactions, the catalytic degradation of nitrophenol and its homologous series have beenwidely considered as one of the most valuable application, not only because of it may causes detrimental effects to the environment and human health, but the reduction of nitrophenol can produce many important industrial raw materials [16]. Seeing that, it is significant to develop suitable catalysts for this reaction as well as promote the development of MOFs, acting as potential heterogeneous catalyst.
Obviously, the rationally design and synthesis more MOFs with diverse structures are an urgent task for the application of MOFs in the field of catalysts. In general, the assembly processes and structures of MOFs are influenced by numerous factors, such as metal ions, organic ligands, solvents and temperature etc., while the choice of the ligand may play the most critical role [17]. Recently, lots of intense research has demonstrated that mixedligand tactics is effective to prepare variegated MOFs, especially those ligands containing carboxyls and N donor groups are more valuable [18]. Within such a context, our group has also prepared a series of MOFs based on this strategy, which show brilliant magnetism, fluorescence and sensing properties [19].
As an extension of the foregoing work, one novel Ag(Ⅰ) metalorganic framework has been prepared successfully by choosing 3, 5-(di(2', 5'-dicarboxylphenyl)benozoic acid (H5ddcba) and 1, 3-di (4-pyridyl)propane (dpp) as pentacaboxylate ligand and N-donor ligand, respectively. Catalytic activity of complex 1 has been demonstrated by its extraordinary performance as a heterogeneous catalyst in the reduction of nitrophenol in aqueous solution.
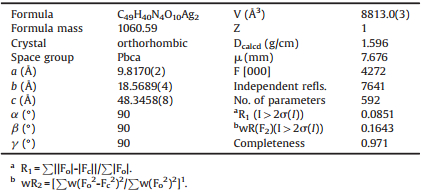
The exact structure of complex 1 was revealed by single-crystal X-ray crystallography. Detailed crystallographic data and refinement are summarized in Table 1. As indicated in Fig. 1, 1 crystallizes in the orthorhombic system with Pbca space group. The minimum asymmetric unit contains two crystallographically independent Ag (I) centers, one complete H3ddcba2- anion and two molecules of dpp. Both Ag 1 and Ag 2 are four-coordinated by two oxygen atoms and two nitrogen atoms (AgN2O2), which are provided by carboxylic group (Ag1-O1 = 2.511(6), Ag1-O10#1 = 2.538(5), Ag2- O1#4 = 2.678, Ag2-O10 = 2.524(5)) and pyridine ring (Ag1- N1 = 2.166(6), Ag1-N3 = 2.176(6), Ag2-N2#2 = 2.178(6), Ag2- N4#3 = 2.186(7)) (Table S1 in Supporting information), respectively. In 1, each H3ddcba2- anion adopts one type of coordinating mode μ4-(η2-μ2)-(η2-μ2) to connect metal ions, while dpp ligand possesses a μ2-(η1:η1) mode. Notably, nitrogen atoms from dpp ligands connect single metal center, resulting in the formation of 1D wavelike chain (Fig. 1c). Further, adjacent chains expand to the 2D wavy layer along ac plane by sharing Ag1 and Ag2 metal clusters. The H3ddcba2- anion shows vital linking functions, bridging the adjacent 2D layers to form a 3D framework eventually, where the nearest nonbonding Ag…Ag distance is 10.294 Å and 10.204 Å. From a topological perspective, every metal cluster is connected with the surrounding six clusters, which could be defined as a 6-connected node. Therefore, the overall structure of 1 is a 3D pcu network with the point symbol of (412·63).
|
|
Table 1 Crystal data and structure refinement of complex 1. |

|
Download:
|
| Fig. 1. (a) Coordination environments of the Ag(Ⅰ) ions in complex 1. Symmetry code: #1 = 1.5-x, -0.5 + y, z; #2 = 1-x, 1-y, -z; #3 = 1-x, 0.5 + y, 0.5-z; #4 = 1.5-x, 0.5 + y, z. (b) The 3D packing structure of 1. (c) Brief illustration of the 1D wavelike chain. (d) Schematic representation and natural tiling of the 6-connected pcu network of 1. | |
The famous framework material MOFs-5 also possesses pcu net in which octahedrally shaped Zn4O(CO2)6 SBUs with six points of extension (carboxylate C atoms) are linked by terephthalate (dicarboxylate) linkers [20]. Compared to compound 1, [Ag2(H3ddcba)(4, 4'-bipy)2], the other Ag(Ⅰ)-MOF that synthesized in our previous work presents ThSi2 (103-b) net, indicating that the configuration of N-donor ligand can produce a profound influence on the final topological network and MOFs structure [21].
To verify the phase purity of the sample, X-ray powder diffraction (PXRD) experiment has been carried out. The main peaks of 2θ = 7.30°, 9.50°, 10.34°, 12.01°, 14.54°, 17.32°, 19.48°, 20.48° and 23.08° in the pattern agree well with the simulated pattern (Fig. S1 in Supporting information). Additionally, thermogravimetric analysis of 1 has been performed in studying its thermal stability. The operation was conducted under O2 atmosphere at heating rate of 10 ℃/min. The TGA curves of complex 1 exhibits two main steps of weight loss, which are attributed to the loss of organic ligands. 1 shows the first loss of 36% below 355 ℃ due to the release of dpp ligand (calcd. 37%). The second step occurs from 340–529 ℃, corresponding to the decomposition of H5ddcba ligand and the formation of Ag2O (calcd. 78% and obsd. 76.2%) (Fig. S2 in Supporting information).
Luminescent properties of metal-organic frameworks which contain d10 metal ions have been attracting more interest because of their potential applications in chemical sensor, photochemistry, and electroluminescent display. For complex 1, excited at 398 nm, it gives rise to an emission band at 490 nm (Fig. S3 in Supporting information). Meanwhile, the maximal emission peak of free H5ddcba ligand appears at 457 nm (λex = 390 nm). Compared with free ligand, the emission band of 1 presents red-shifts, which should be ascribed to the metal-ligand coordinative interactions [22].
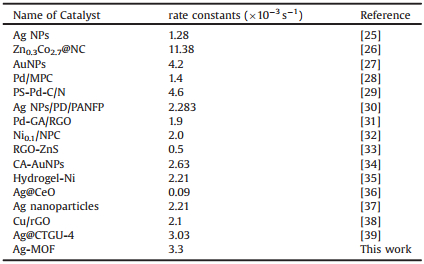
Inspiring by previous work that Ag-MOF has been successfully used as catalytic material, we also explored the application of complex 1 in reduction of nitrophenol. Beyond the significance that mentioned in the introduction, the degradation of nitrophenol in aqueous solutions is often chosen as a model reaction to evaluate the catalytic performance, due to the convenience of absorbance signal monitoring. Generally, the aqueous solution of 4-NP, 2-NP and 3-NP show a strong absorption peak at 317, 277 and 272 nm. After the addition of KBH4, the absorption peak of nitrophenol red-shifted to 400, 390 and 414 nm (Fig. S5 in Supporting information), simultaneously the color of solution turned to yellow-green due to the formation of nitrophenolate ions [23]. Then, each of the three nitroaromatics exhibits a negligible change, indicating that KBH4 itself has no catalytic activity for the reaction. Upon the addition of desired amount of complex 1, the absorption of substrates significantly decreases at monitored wavelength. Finally, three nitroaromatics were decomposed completely in the presence of 1 and KBH4 within the time of 11–14 min (Fig. 2). To better understand the catalytic processes, the kinetics of the reduction was further investigated. Because of the high initial concentration of KBH4 solution (C(KBH4)/C (NP) = 16–71), the above catalytic reaction followed the Langmuir-Hinshelwood apparent first order kinetics model, of which kinetics was ln(Ct/C0) = kt (k is apparent rate constant, t is reduction time, Ct and C0 is concentrations of nitrophenol) [24]. As shown in Fig. 2 (inset), in each case, the linear relationship between ln(Ct/C0) and reaction time is obtained. The reaction rate constants were calculated to be 3.3×10-3 s-1 (4-NP), 3.1×10-3 s-1 (2-NP) and 1.9×10-3 s-1 for 3-NP. According to the literature, the silver nanoparticles that were fabricated in aqueous solution by using micellar assemblies of dendritic amphiphiles containing triazole rings also present outstanding activities in this reaction, whose parameter is 1.28×10-3 s-1 [25]. It is worth noting that the rate of catalytic reaction was higher in case of [Ag2(ddcba)(4, 4'-bipy)2] (7.6×10-3 s-1 for 4-NP), indicating that different coordination environment might be affect final properties. Furthermore, Table 2 displays a comparison of the catalytic activities in our work with previous reports [26-39].

|
Download:
|
| Fig. 2. UV–vis spectra showing gradual reduction of 4-NP (a), 2-NP (b) and 3-NP (c) over complex 1. Inset: the relationship between ln(Ct/C0) and reaction time (t). | |
|
|
Table 2 Summary of rate constants of other similar 4-nitrophenol reduction reactions catalyzed by previously reported catalysts. |
In summary, a novel metal-organic framework with Ag node, pentacarboxylate linker and N-donor ligand, which features a 3D framework with a 6-connected pcu topology, has been constructed under hydrothermal condition and the as-prepared material can be applied as an effective catalyst for the reduction of nitrophenol. The present work points out that Ag-MOFs are expected to play an important role in the applications such as heterogeneous catalytic hydrogenation of various nitrophenols and other similar reactions. Meanwhile, it provides a potential way to the selective design of silver-based crystalline materials, combing with our previous work.
AcknowledgmentWe appreciate the financial support from the National Science Foundation of China (Nos. 216731272, 2137312, 21671119, 51572152 and 51502155).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.09.043.
CCDC 1565060 contains the supplementary crystallographic data for complex 1. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
| [1] |
H.C. Zhou, S. Kitagawa, Chem. Soc. Rev. 43 (2014) 5415-5418. DOI:10.1039/C4CS90059F |
| [2] |
H.C. Zhou, J.R. Long, O.M. Yaghi, Chem. Rev. 112 (2012) 673-674. DOI:10.1021/cr300014x |
| [3] |
D.S. Li, Y.P. Wu, J. Zhao, J. Zhang, J.Y. Lu, Coord. Chem. Rev. 261 (2014) 1-27. DOI:10.1016/j.ccr.2013.11.004 |
| [4] |
Y.P. Wu, W. Zhou, J. Zhao, et al., Angew. Chem. Int. Ed. (2017). DOI:10.1002/anie.201707238 |
| [5] |
Y.F. Chen, X.Q. Huang, S.H. Zhang, et al., J. Am. Chem. Soc. 138 (2016) 10810-10813. DOI:10.1021/jacs.6b06959 |
| [6] |
Q.H. Yang, Q. Xu, S.H. Yu, H.L. Jiang, Angew. Chem. Int. Ed. 55 (2016) 3685-3689. DOI:10.1002/anie.201510655 |
| [7] |
J.D. Xiao, Q.H. Shang, Y.J. Xiong, et al., Angew. Chem. Int. Ed. 128 (2016) 9535-9539. DOI:10.1002/ange.201603990 |
| [8] |
B. Wang, H. Yang, Y.B. Xie, et al., Chin. Chem. Lett. 27 (2016) 502-506. DOI:10.1016/j.cclet.2015.12.034 |
| [9] |
O. Alduhaish, B. Li, H. Arman, et al., Chin. Chem. Lett. 28 (2017) 1653-1658. DOI:10.1016/j.cclet.2017.04.025 |
| [10] |
M. Yoon, R. Srirambalaji, K. Kim, Chem. Rev. 112 (2012) 1196-1231. DOI:10.1021/cr2003147 |
| [11] |
Q.H. Yang, Q. Xu, H.L. Jiang, Chem. Soc. Rev. 46 (2017) 4774-4808. DOI:10.1039/C6CS00724D |
| [12] |
G.W. Xu, Y.P. Wu, W.W. Dong, et al., Small 13 (2017) 1602996. DOI:10.1002/smll.v13.22 |
| [13] |
J.C. Wang, J.P. Ma, Q.K. Liu, Y.H. Hu, Y.B. Dong, Chem. Commun. 52 (2016) 6989-6992. DOI:10.1039/C6CC00576D |
| [14] |
A.Q. Ma, L.G. Zhu, RSC Adv. 4 (2014) 14691-14699. DOI:10.1039/C3RA47136E |
| [15] |
K.M. Betancor, S. Aguado, I.R. Palomares, et al., Sci. Total Eviron. 595 (2017) 547-555. DOI:10.1016/j.scitotenv.2017.03.250 |
| [16] |
N. Pradhan, A. Pal, T. Pal, Langmuir 17 (2001) 1800-1802. DOI:10.1021/la000862d |
| [17] |
M. Roy, S. Sengupta, S. Bala, S. Bhattacharya, R. Mondal, Cryst. Growth Des. 16 (2016) 3170-3179. DOI:10.1021/acs.cgd.5b01835 |
| [18] |
M. Du, C.P. Li, C.S. Liu, S.M. Fang, Coord. Chem. Rev. 257 (2013) 1282-1305. DOI:10.1016/j.ccr.2012.10.002 |
| [19] |
(a) D. S. Li, J. Zhao, Y. P. Wu, et al., Inorg. Chem. 52(2013) 8091-8098; (b) D. S. Li, P. Zhang, J. Zhao, et al., Cryst. Growth Des. 12(2012) 1697-1702; (c) C. Li, D. S. Li, J. Zhao, et al., CrystEngComm 13(2011) 6601-6609. |
| [20] |
M. Li, D. Li, M. O'Keeffe, O.M. Yaghi, Chem. Rev. 114 (2014) 1343-1370. DOI:10.1021/cr400392k |
| [21] |
X.Q. Wu, G.X. Wen, Y.P. Wu, et al., J. Solid State Chem. 242 (2016) 243-247. DOI:10.1016/j.jssc.2016.08.001 |
| [22] |
L. Liu, J. Ding, C. Huang, et al., Cryst. Growth Des. 14 (2014) 3035-3043. DOI:10.1021/cg500295r |
| [23] |
D. Xu, D.L. Zhang, H.B. Zou, et al., Chem. Commun. 52 (2016) 10513-10516. DOI:10.1039/C6CC05366A |
| [24] |
C. Kastner, A.F. Thunemann, Langmuir 17 (2001) 1800-1802. DOI:10.1021/la000862d |
| [25] |
N.G. Patil, N.B. Basutkar, A.V. Ambade, New J. Chem. 41 (2017) 4546-4554. DOI:10.1039/C7NJ00605E |
| [26] |
X.J. Xu, H. Li, H.T. Xie, Y.H. Ma, J. Mater. Res. 32 (2017) 1777-1786. DOI:10.1557/jmr.2017.148 |
| [27] |
Y. Dai, T. Ren, Y. Wang, X.J. Zhang, Gold Bull. 50 (2017) 123-129. DOI:10.1007/s13404-017-0204-1 |
| [28] |
Z.P. Dong, X.D. Le, Y.S. Liu, C.X. Dong, J.T. Ma, J. Mater. Chem. A 2 (2014) 18775-18785. DOI:10.1039/C4TA04010D |
| [29] |
Y. Long, Y.S. Liu, Z.M. Zhao, et al., J. Colloid Interface Sci. 496 (2017) 465-473. DOI:10.1016/j.jcis.2017.02.051 |
| [30] |
S.X. Lu, J.Y. Yu, Y.Y. Cheng, et al., Appl. Surf. Sci. 411 (2017) 163-169. DOI:10.1016/j.apsusc.2017.03.120 |
| [31] |
A.T. Vilian, S.R. Choe, K. Giribabu, et al., J. Hazard. Mater. 333 (2017) 54-62. DOI:10.1016/j.jhazmat.2017.03.015 |
| [32] |
Y. Yang, Y. Zhang, C.J. Sun, et al., Cat. Chem. 6 (2014) 3084-3090. |
| [33] |
S. Ibrahim, S. Chakrabarty, S. Ghosh, T. Pal, Chem. Select 2 (2017) 537-545. |
| [34] |
Y.S. Seo, E.Y. Ahn, J. Park, et al., Nanoscale Res. Lett. 12 (2017) 7. DOI:10.1186/s11671-016-1776-z |
| [35] |
J.Z. Ding, Q. Li, L.W. Zhao, et al., RSC Adv. 7 (2017) 17599-17611. DOI:10.1039/C7RA01077J |
| [36] |
Y.Y. Wang, Y. Shu, J. Xu, H. Pang, CrystEngComm 19 (2017) 684-689. DOI:10.1039/C6CE02165D |
| [37] |
J. Safari, Z. Zarnegar, M. Sadeghi, A.E. Najafabadi, J. Mol. Strust. 1125 (2016) 772-776. DOI:10.1016/j.molstruc.2016.07.056 |
| [38] |
K. Żelechowska, I. Kondratowicz, Pol. J. Chem. Technol. 18 (2016) 47-55. |
| [39] |
X.Q. Wu, D.D. Huang, Z.H. Zhou, et al., Dalton Trans. 46 (2017) 2430-2438. DOI:10.1039/C7DT00024C |
 2018, Vol. 29
2018, Vol. 29