b Key Laboratory of Cluster Science Ministry, Ministry of Education of China, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing 100081, China
Rechargeable lithium-ion batteries (LIBs) have become the dominant power sources for portable electronics such as mobile phones, laptop computers, digital cameras, videos, etc. Targeted to powering future electric vehicles and applying in large-scale electric energy storage, the energy density of a LIB is one of the key parameters to be enhanced, in addition to the cost and safety issues [1]. As for the anode material of a LIB, graphite is currently applied due to its high coulombic efficiency and cycling performance. However, its low theoretical capacity (372 mAh/g) and limited rate capability do not meet the demand in future applications [2]. Therefore, to develop an anode material with a higher capacity to replace graphite is of great research interest for next-generation LIBs. Materials that undergo a conversion reaction upon lithiation/ delithiation are able to provide an enhanced theoretical capacity, such as alloys and transition metal oxides. These materials have low cost, abundant and are environmentally benign. However, arising from the large volume variation in the electrochemical process and poor conductivity become the major obstacles to practical application [3]. Many efforts have been attempted to develop a new anode material combining graphite and metal component, but the result is unsatisfying to date. Indeed, developing materials with high energy density, rapid capacity and long cycling performance is still challenging [4].
Metal-organic frameworks (MOFs) have emerged as a class of crystalline porous materials yielded from the coordination of metal ions and organic linkers that can provide rich porosity with very high surface areas. The extraordinary degree of variability for both the organic and inorganic components of MOFs and their properties, make MOFs of great interest for potential applications in many fields [5-7]. Meanwhile, MOFs have been proved to be an ideal sacrificial template and reactive precursor for the fabrication of various carbon-based nanomaterials. It is possible to synthesize metal nanoparticles and metal oxide nanostructures that are highly dispersed on or embedded in a highly conductive porous carbon matrix via thermal pyrolysis under a controlled atmosphere. Such nanostructures are able to improve the storage capacity of Li+ and increase the transport of Li+ as well, leading to anode materials with both higher capacity and rate capability [8, 9].
Appropriate selection of the MOFs precursor is crucial for the performance of the resultant carbon-based composite. Although several anode materials derived from MOFs have been reported [10-13], the pyrolysis products mostly contained single metal component and their performance as anode materials were unsatisfactory. Two anode materials were derived from bimetallic MOFs, e.g., Zn/Co-MOF-5 and Co/Ni-MOF-74 [14, 15], but their metal components were low owing to the low crystallographic density of the chose MOF precursors (e.g., MOF-74: 0.45 g/cm3), which would lead to low volumetric capacity densities. Therefore, to choose a bimetallic MOF with a higher density of metal component would help to improve the volumetric capacity density of the resultant anode material.
Zeolite imidazolate frameworks (ZIFs) are a famous subclass of MOFs in which divalent metal cations are linked with imidazolate anions into tetrahedral frameworks that possess a zeolite topology [16, 17]. ZIFs possess high crystallography density and can yield abundant metal sites in the carbon nanocomposite after pyrolysis. Herein, we selected the nickel-substituted ZIF-8 synthesized by our group (denoted as Zn/Ni-ZIF-8) as the precursor to produce bimetallic nitrogen-doped porous nanocomposites [18]. The MOF possesses a high crystallographic density of 1.0 g/cm3, its Ncontaining 2-methylimidazole ligand and well-dispersed metal clusters (zinc and nickel) make it an ideal precursor to give nanocomposites with improved volumetric capacity density as anode materials [19].
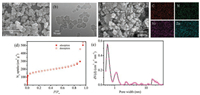
Zn/Ni-ZIF-8 was synthesized by the method of literature [18]. The pyrolysis procedures of Zn/Ni-ZIF-8 were conducted in a horizontal quartz reactor under an N2 flowing atmosphere. The calcination procedures of ZIF-8 were conducted under the same conditions. Powder X-ray diffraction (PXRD) pattern shows the amorphous nature of Zn/Ni-ZIF-8-800 and displays two broad peaks around 25° and 44°(Fig. S2 in Supporting information), corresponding to carbon (002) and (101) diffractions, respectively. Scanning electron microscope (SEM) and transmission electron microscopy (TEM) suggest that the obtained Zn/Ni-ZIF-800 retains the original morphology of the MOF prepursor (Figs. 1a and b). The size of the particles is about 50–200 nm. Elemental mapping reveals that metal elements are uniformly dispersed in the carbon matrix (Fig. 1c). N2 adsorption–desorption isotherms of Zn/Ni-ZIF- 8-800 at 77 K reveals an adsorption curve close to type I with a Brunauer-Emmett-Teller (BET) surface area of 620 m2/g (Fig. 1d). Pore-size distribution analysis based on nonlocal density functional theory (NLDFT) method indicates that the pores of Zn/NiZIF-8-800 are most in the micro-, meso-porous regions (Fig. 1e). Most micropores were remained and some mesopores appeared. The wide pore size distribution was attributed to metalimidazolate coordination broke caused by partial evaporation of Zn during the pyrolysis. The nanopores provide more favourable pathways for Li+ penetration and transport. Therefore, Zn/Ni-ZIF- 8-800 is suggested to be an excellent anode material for LIBs.
|
Download:
|
| Fig. 1. (a) SEM image; (b) TEM image; (c) EDX elemental mapping of Zn/Ni-ZIF-8-800; (d) Nitrogen (N2) adsorption/desorption isotherms at 77 K; (e) Pore size distributions of Zn/Ni-ZIF-8-800. | |
X-ray photoelectron spectroscopy (XPS) was used to further investigate the nature of nitrogen and metal species on the surface of Zn/Ni-ZIF-8-800 (Figs. S3-S6 in Supporting information). Two intense peaks at 398.3 eV and 400.4 eV can be distinguished, the former corresponding to pyridinic nitrogen, and the latter to pyrrolic-N [20, 21]. The small peak at 402.0 eV may be attributed to quaternary-N, which is often observed in the case of the nitrogen species under pyrolysis conditions. The peaks at 1021.9 eV and 1044.9 eV of Zn 2p 2/3 and 2p 1/2 can also be identified [22]. The Ni peaks at 871.8 eV and 855.0 eV may be attributed to Ni 2p 2/3 [23].
Elemental analysis results (Table 1) confirm that Zn/Ni-ZIF-8- 800 is composed of C, H, N, Zn, and Ni. Because the calcination temperature (800 ℃) is close to the boiling point of Zn (906 ℃) but far below to the boiling point of Ni (2730 ℃), the ratio of Zn/Ni is lower in Zn/Ni-ZIF-8-800 than that in the original Zn/Ni-ZIF-8.
|
|
Table 1 The comparison of elemental analyses for Zn/Ni-ZIF-8 and Zn/Ni-ZIF-8-800. |
CR2032 coin cells with metallic lithium as counter electrode were assembled to evaluate the performance of Zn/Ni-ZIF-8-800. Fig. 2a shows the cycling performance of Zn/Ni-ZIF-8-800 at a high current rate of 500 mA/g. The discharge capacity is 1912.1 mAh/g in the first cycle and retains at 1105.2 mAh/g after 400 cycles. The initial Coulombic efficiency is 66%, attributed to the formation of a solid electrolyte interphase (SEI), which is often observed for anode materials. Fig. 2b shows the voltage profiles of Zn/Ni-ZIF-8- 800, exhibiting sloping curves typical for transition metal oxides. The conversion reaction is highly reversible as suggested by the nearly identical voltage curves over hundreds of cycles, the cathodic/anodic potentials are constant and in consistence with the cyclic voltammetry (CV) results (Fig. S7 in Supporting information).

|
Download:
|
| Fig. 2. (a) Discharge and charge capacity of Zn/Ni-ZIF-8-800 electrodes over 400 cycles at 500 mA/g; (b) Corresponding voltage profiles; (c) Cycling performance of Zn/Ni-ZIF- 8-800 and ZIF-8-800 over 150 cycles at 500 mA/g; (d) Comparison of the reversible capacity of commercial graphite, ZIF-8-800 and Zn/Ni-ZIF-8-800. | |
The electrochemical performance of Zn/Ni-ZIF-8-800 was compared with ZIF-8-800, obtained from monometallic ZIF-8 under identical pyrolitic conditions. Fig. 2c shows that the discharge capacity of ZIF-8-800 is 467.0 mAh/g after 150 cycles at 500 mA/g, much lower than that of Zn/Ni-ZIF-8-800 (1111.4 mAh/g) (Fig. 2c). The reversible discharge capacity of Zn/Ni-ZIF-8- 800 is about 200% increase compare with that of commercial graphite (Fig. 2d).
Generally, metal species can significantly improve the electrochemical activity of materials for the good conductivity. Meanwhile, metal and its composite can significantly improve the contact among the active particles and between active particles and collection of fluid, resulting in improving the electronic circulation ability. Therefore, the electrochemical performance of the material doped Zn and Ni metal species is much better than carbon materials. As for Zn/Ni-ZIF-8 and ZIF-8, although they have the similar structures, the pyrolysis product has Ni species of the former as starting material and the forming nanoporous carbon also have more defects to attract more Li+. Zn and Ni can form alloy easily to bring better electronic transmission capacity and provide more Li+ embedding sites. Moreover, it suggests that the electro potential of Ni (E(Ni2+/Ni) = -0.257 V) is higher than that of Zn (E (Zn2+/Zn) = -.76 V). Ni2+ will be redox more easily than Zn2+ under the condition of the high temperature of 800 ℃ to generate good crystallinity metal nanoparticles, thus the electrochemical performance of Zn/Ni-ZIF-8-800 is better than that of ZIF-8-800.
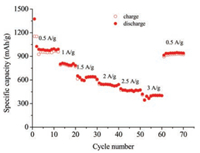
Fig. 3 presents the rate capabilities at various current rates (from 0.5 A/g to 3 A/g). When the current rate gradually increased from 0.5 A/g to 1, 1.5, 2, 2.5 and 3 A/g, the corresponding discharge capacities are 987.9, 835.8, 628.3, 541.0, 469.0 and 400.1 mAh/g, respectively. Even at 3 A/g, the reversible capacity is still greater than the theoretical capacity of graphite (372 mAh/g). When the current rate decreased from 3 A/g to 0.5 A/g, the discharge capacity recovers to 940.1 mAh/g, indicating good robustness of the anode material. The excellent rate performance of Zn/Ni-ZIF-8-800 can be contributed to its nanoporous structure for accommodating the volume change upon cycling while facilitating the diffusion of Li+ [9, 24].
|
Download:
|
| Fig. 3. Specific capacities at current densities ranging from 0.5 A/g to 3 A/g. | |
In summary, a highly stable and in situ bimetal- and nitrogendoped porous composite derived from a novel bimetal-organic framework were successfully prepared using a one-step controlled pyrolysis. By virtue of the nanocarbon and highly spread bimetal active components via pyrolysis, Zn/Ni-ZIF-8-800 exhibited an excellent rate capacity, cyclic reversibility and long cyclic life as an anode material for LIBs. The composite delivers a greater discharge capacity of 1105.2 mAh/g at a current density of 500 mA/g after 400 cycles. Such a bimetalic nanocomposite can provide the benefit of accommodating the volume change and facilitating the diffusion of Li+ during the discharge-charge process when being applied as an anode material for LIBs. The selection of a bimetalic MOF as precursor as well as the simple synthetic process may promote the design of novel electrode materials with smarter nanostructures and better performance for next-generation LIBs. This may shed light on developing new material derived from metal-organic frameworks for energy applications.
AcknowledgmentsThis work was financially supported by the 973 Program (No. 2013CB834704), the National Natural Science Foundation of China (Nos. 21471018, 21404010, 21201018, 21490570), and 1000 Plan (Youth).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.09.024.
| [1] |
J.M. Tarascon, M. Armand, Nature 414 (2001) 359-367. DOI:10.1038/35104644 |
| [2] |
P. Ridgway, H. Zheng, A.F. Bello, et al., Electrochem. Soc. 159 (2012) 520-524. DOI:10.1149/2.006205jes |
| [3] |
M.V. Reddy, G.V. Subba Rao, B.V. Chowdari, Chem. Rev. 113 (2013) 5364-5457. DOI:10.1021/cr3001884 |
| [4] |
B. Scrosati, J. Hassoun, Y.K. Sun, Energy Environ. Sci 4 (2011) 3287-3295. DOI:10.1039/c1ee01388b |
| [5] |
H. Zhou, J.R. Long, O.M. Yaghi, Chem. Rev. 112 (2012) 673-674. DOI:10.1021/cr300014x |
| [6] |
J.R. Long, O.M. Yaghi, Chem. Soc. Rev. 38 (2009) 1213-1214. DOI:10.1039/b903811f |
| [7] |
H. Furukawa, K.E. Cordova, O'Keeffe M., O.M. Yaghi, Science 341 (2013) 974-988. |
| [8] |
A.D. Roberts, X. Li, H. Zhang, Chem. Soc. Rev. 43 (2014) 4341-4356. DOI:10.1039/C4CS00071D |
| [9] |
S. Xin, Y. Guo, L. Wan, Acc. Chem. Res. 45 (2012) 1759-1769. DOI:10.1021/ar300094m |
| [10] |
F. Zou, Y. Chen, K. Liu, et al., ACS Nano 10 (2016) 377-386. DOI:10.1021/acsnano.5b05041 |
| [11] |
G. Zhang, S. Hou, H. Zhang, et al., Adv. Mater. 27 (2015) 2400-2405. DOI:10.1002/adma.v27.14 |
| [12] |
R. Wu, D.P. Wang, X. Rui, et al., Adv.Mater. 27 (2015) 3038-3044. DOI:10.1002/adma.201500783 |
| [13] |
P. Wang, J. Lang, D. Liu, X. Yan, Chem. Commun. 51 (2015) 11370-11373. DOI:10.1039/C5CC01703C |
| [14] |
C. Li, X. Hu, X. Lou, Q. Chen, B. Hu, Chem. Commun. 52 (2016) 2035-2038. DOI:10.1039/C5CC07151H |
| [15] |
H. Li, M. Liang, W. Sun, Y. Wang, Adv. Funct. Mater. 26 (2016) 1098-1103. DOI:10.1002/adfm.v26.7 |
| [16] |
K.S. Park, Z. Ni, A.P. Côté, et al., Proc. Natl. Acad. Sci. U. S. A. 103 (2006) 10186-10191. DOI:10.1073/pnas.0602439103 |
| [17] |
A. Phan, C.J. Doonan, F.J. Uribe-Romo, et al., Acc. Chem. Res. 43 (2010) 58-67. DOI:10.1021/ar900116g |
| [18] |
R. Li, X. Ren, H. Ma, et al., J. Mater. Chem. A 2 (2014) 5724-5729. DOI:10.1039/C3TA15058E |
| [19] |
R. Li, X. Ren, X. Feng, et al., Chem. Commun. 50 (2014) 6894-6897. DOI:10.1039/c4cc01087f |
| [20] |
W. Li, Z. Zhang, B. Kong, et al., Angew. Chem. Int. Ed. 52 (2013) 8151-8155. DOI:10.1002/anie.v52.31 |
| [21] |
S. Feng, W. Li, Q. Shi, et al., Chem. Commun. 50 (2014) 329-331. DOI:10.1039/C3CC46492J |
| [22] |
D. Owen, D.G. Zetaruk, N.S. Mcintyre, J. Electrochem. Soc. 126 (1979) 750-760. DOI:10.1149/1.2129132 |
| [23] |
M.C. Biesinger, L.W.M. Lau, A.R. Gerson, R.S.C. Smart, Appl. Surf. Sci. 257 (2010) 2717-2730. |
| [24] |
Y. Guo, J. Hu, L. Wan, Adv. Mater. 20 (2008) 2878-2887. DOI:10.1002/adma.v20:15 |
 2018, Vol. 29
2018, Vol. 29 



