Cancer has become one of the most serious diseases of threatening human health all over the world. However, conventional therapy of anticancer drugs is lack of specificity in tumor sites which often causes systematic toxicity and limits clinical application. In the recent past decades, biodegradable nanoparticles and micelles have been extensively developed to control and target delivery of anticancer drugs for enhancing therapeutic efficacy and reducing their anticancer adverse effects [1-4]. These carriers have exhibited many beneficial properties such as better pharmacological profiles, decreasing adverse effects, enhancing drug accumulation in the tumor sites, prolonging blood circulation time as well as improving drug tolerance [5-7]. It is noted, however, the amount of anticancer drugs in the intracellular compartments of cancer cells is usually too inadequate to kill cancer cells owing to low release doses or slow release rates of anticancer drug from polymeric micelles [8, 9]. Recently, a variety of stimuli-trigger systems including pH [10, 11], enzymes [12], temperature [13], in particular reduction-sensitive nanocarriers [14-16], have attracted special attention and been explored for improving cancer therapy. Disulfide bonds can remain stable under normal physiological conditions (pH 7.4) whereas they are rapidly cleaved via thiol-disulfide reaction especially with glutathione (GSH), an abundant thiol species in the cytoplasm, especially within tumor cells [17].
Bletilla striata polysaccharides (BSP) produced by the tubers of Bletilla striata (Thunb.) Reichb.f. have a backbone structure consisting of β (1→4) linked mannose and glucose with molar ratio of 3:1 and have be applied to control drug delivery due to good biocompatibility [18] and biodegradability [19]. However, BSP have a deficiency of sustaining drug release because of its hydrophilic property.
Herein, a reduction-sensitive stearic acid (SA) grafted-Bletilla striata polysaccharide (BSP-ss-SA) copolymer was synthesized using cystamine as a linkage arm through three synthesis steps to overcome this limitation and characterization was also carried out. The synthetic route of BSP-ss-SA conjugates was illustrated in Fig. S1 in Supporting information. First, succinic anhydride serving as a connecting arm was conjugated to BSP to achieved BSP-COOH. Second, BSP-CYS was synthesized through linking cystamine (CYS) and BSP-COOH. Third, amidation reaction between carboxyl groups of stearic acid (SA) and amine group of BSP-CYS was polymerized to synthesize BSP-ss-SA copolymer.
Fourier transform infrared (FTIR) spectra were recorded on Shimadzu 8300 FTIR spectrometer (Shimadzu) and 1H nuclear magnetic resonance (1H NMR) spectra were recorded on a 400 MHz NMR spectrometer (AVⅢ, Bruker) for identifying the structure. The FTIR spectra were presented in Fig. S2 in Supporting information. The characteristic band at 3400 cm-1 was attributed to the characteristic absorption of the O—H stretching vibration in BSP (Fig. S2-A). Moreover, the absorption peak at 1629.74 cm-1 was assigned to stretching vibration of intra-molecular hydrogen bond. These signals suggested there were plenty of hydroxyl groups in BSP. The absorption band at 2931.60 cm-1 was ascribed to C—H stretching vibration of methylene (—CH2—) in BSP, 1024.13 cm-1 and 1153.35 cm-1 corresponded to the C—O—C stretching vibration in BSP. The two peaks at 874 cm-1 and 815 cm-1 were attributed to β-glucosyl and mannose residues, respectively [20]. Succinic anhydride has been conjugated onto BSP and BSP-COOH have been succussfully synthetized as revealed the detection of a new wide peak at 1733.99 cm-1 corresponding to the stretching vibration of C=O (Fig. S2-B). In Fig. S2-C, it was worth mentioning that these new peaks at 1731.96 cm-1, 1645.17 cm-1 and 1573.81 cm-1 were attributable to the stretching vibration of C=O (—O—C=O), C=O (—NH—C=O) and N—H, respectively, suggesting the existence of ester and amide groups. These signals demonstrated BSP- CYS have been achieved. The FTIR spectrum of BSP-ss-SA copolymer (Fig. S2-D) was similar with that of BSP-CYS. However, the peak intensity at 1573.81 cm-1 of BSP-ss-SA was stronger than that of BSP-CYS (1569.93 cm-1), indicating the change of dipole moment and vibration mode to some extent when SA was conjugated to BSP-CYS. 1H NMR spectra were presented in Fig. S3 in Supporting informatinon. The series of peaks at δ 3.14 ~ 4.19 ppm were assigned to hydroxyl groups in BSP (Fig. S3-A). The single peak at δ 4.93 ppm (H2) arose from β (1→4) linked hydrogen proton and the peaks at around δ 5.37 ppm (H1) were assigned to β (1→6) linked hydrogen protons of BSP. The results were in accordance with our previous result [21]. The peaks at δ 2.54 ~ 2.82 ppm (H3, H4) were attributed to hydrogen protons of methylene (C—CH2—CH2—C) of succinic anhydride in BSP-COOH (Fig. S3-B). For BSP-CYS (Fig. S3-C), the signals at δ 2.99 ppm (H6) and δ 3.36 ppm (H5) were assigned to the protons of methylene (NH—CH2—CH2—S) of cystamine. In Fig. S3-D, two new peaks appeared at δ 0.87 ppm (H8) and δ 1.28 ppm (H7) were assigned to the hydrogen proton of methylene and methyl proton (CO—(—CH2)16—CH3) of SA, respectively. The 1H NMR spectra could regarded as another evidence for successful synthesis of BSP-ss-SA. The substitution of degree (DS) succinic anhydride onto BSP was approximate 46.0% measured by acid-base titration. The substitution degree of CYS on BSP-COOH or the substitution degree of SA on BSP-CYS calculated by 1H NMR analysis was 36.5% and 1.06%, respectively.
The particle size distribution as well as zeta potential of micelles were determined on a dynamic light scattering (DLS) particle size analyzer (Zetasizer Nano ZS, Malvern Instruments, UK) in aqueous media. The results were presented in Fig. S4 in Supporting information. BSP-ss-SA copolymers could selfassemble into micelles with a mean diameter of (106±4.36) nm and many negative surface charges. The changes of particle size and zeta potential within 15-day storage at room temperature were evaluated and showed in Fig. S4. Particle size showed slow increase trend as storage time prolong and particle size became a bit larger after 7-day storage whereas no change of zeta potential was seen. The increase of particle size might be due to huge specific surface areas and surface energy of micelle systems, resulting in spontaneous particles aggregation. However, the phenomenon was a physical change not a chemical change. Therefore, no change of zeta potential was found. Dithiothreitol (DTT) was a potent reducing agent and exhibited a strong reduction ability to destroy disulfide bonds of some bio-macromolecules. Therefore, DTT was usually chosen to replace GSH in vitro experiments [22-24]. The changes of particle size were monitored in response to 0 and 10 mmol/L DTT at (37±0.5) ℃ and results were offered in Fig. 1. Almost no distinct change of particle size was found over 24 h in pH 7.4 PBS in absence of DTT whereas the average diameter of micelles distinctly increased from 106 nm (0 h) to 396 nm and 2300 nm (24 h) in the presence of DTT within 24 h. The phenomenon may be attributed to the cleavage of disulfide bonds [25].

|
Download:
|
| Fig. 1. Particle size change. A represents the change of particle size in the presence of DTT; B represents the change of particle size change with or without 10 mmol/L DTT at (37±0.5) ℃. | |
Fluorescence method using pyrene as a fluorescence probe is often used to measure the aggregation behavior of amphiphilic polymers [26].The final concentration of pyrene was adjusted to 6.0 × 10-7 mol/L. The profile of fluorescence intensity ratio (I374/I384) versus the concentration logarithm of BSP-ss-SA copolymer was plotted in Fig. S5 in Supporting information. The value of critical aggregation concentration (CAC) was 76.36 μg/mL, which was relatively high compared with that similar materials in some literatures [23, 25]. We have ever attempted to obtain lower CAC value of BSP-ss-SA copolymer by increasing the amount of stearic acid in the reaction solution. But BSP-ss-SA copolymer displayed a poor solubility even in dimethyl sulfoxide (DMSO) with the amount of SA increased, which was difficult to further prepare micelles.
Docetaxel (DTX)-loaded micelles were prepared referring to the description in our previous report [21]. Drug loading content (LC) and encapsulation efficiency (EE) were determined at 230 nm by High performance liquid chromatography (HPLC) using acetonitrile and distilled water (V/V, 50:50) as mobile phase and were calculated according to the Eqs. (1) and (2). The LC and EE were 5.94 wt% and 72.75 wt%, respectively.
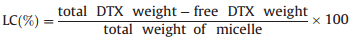
|
(1) |

|
(2) |
PBS (pH 7.4) containing 0.2% of Tween 80 with or without 10 mmol/L DTT was selected to simulate drug release in human blood and the tumor extracellular and intracellular compartments. DTX release rates in vitro were carried out using a dialysis method. 1 mL of the release medium was withdrawn at predetermined time intervals. Meanwhile, an equal temperature and equal volume of fresh medium was immediately compensated. All samples were analyzed by HPLC as above mention after filtrating via 0.45 μm filtration membrane. All experiments were conducted in triplicate. The drug accumulated release percentage verse time was plotted as shown in Fig. 2.

|
Download:
|
| Fig. 2. In vitro release profiles of DTX injection and DTX-loaded micelle with and without DTT in pH 7.4 PBS containing 0.2% Tween 80 at (37 ± 0.5) ℃, respectively (mean±SD, n = 3). | |
The rank of DTX accumulative release amount was DTX injection (0 mmol/L DTT).≈DTX injection (10 mmol/L DTT) > DTX-loaded micelle (10 mmol/L DTT) > DTX-loaded micelle (0 mmol/L DTT). No distinct release difference was observed between DTX injection with and without 10 mmol/L DTT (P > 0.05) during the whole release procedure (48 h) whereas a remarkable release difference was found between DTX injection and DTXloaded micelle (P < 0.01). Moreover, it was obvious that there was no significant difference in the first 5 h (P > 0.05) whereas a significant difference was observed from 5 h to 48 h (P < 0.05) between DTX-loaded micelle in the presence of 10 mmol/L DTT and in the absence of 10 mmol/L DTT. DTX release from micelle showed a rapid release before first 12 h and then maintained a stable release behavior. The result was probably attributed to the cleavage of the disulfide bond in BSP-ss-SA and destabilization of micelles.
The cytotoxicity of BSP-ss-SA copolymer against HepG2 cells was investigated by MTT assay and was presented in Fig. S6 in Supporting information. Cell viability rates were over 85% even at the concentration of 40 μg/mL which illustrated BSP-ss-SA copolymer possessed a good biocompatibility.
Anticancer activity in vitro of DTX-loaded micelles and DTX injection were conducted using MTT assay. The results were shown in Fig. S7 in Supporting information. The anticancer activity of DTX-loaded micelles or DTX injection showed an increase trend with the increase of DTX concentration ranged from 0.0005 μg/mL to 0.5 μg/mL. In addition, the anticancer activity of DTX-loaded micelles was significantly superior to that of DTX injection, especially at DTX concentration of 0.05 μg/mL or 0.5 μg/mL (P < 0.01), which was probably due to the endocytosis of micelles.
In this study, a reduction-sensitive BSP-ss-SA copolymer was successfully synthesized via esterification and amidation reaction. BSP-ss-SA copolymer could spontaneously self-assembled into nanostructure micelle and displayed more rapid drug release rate under reduction environment compared with non-reduction environment. These results suggested that BSP-ss-SA copolymer might be a promising candidate as a nanocarrier for anticancer drug delivery.
AcknowledgmentThe research is supported by Health and Family Planning Commission of Jilin Province (No. 2017J056).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.02.013.
| [1] |
Q. Chen, J.W. Zheng, X.Z. Yuan, J.F. Wang, L.J. Zhang, J. Mater. Sci. Eng. C 82 (2018) 1-9. DOI:10.1016/j.msec.2017.08.026 |
| [2] |
B.K. Qin, L. Liu, X.H. Wu, et al., Acta Biomaterialia 64 (2017) 211-222. DOI:10.1016/j.actbio.2017.09.040 |
| [3] |
L.P. Qiu, M.Q. Zhu, K. Gong, et al., Mater. Sci. Eng. C 78 (2017) 912-922. DOI:10.1016/j.msec.2017.04.137 |
| [4] |
H.L. Gao, Acta Pharm. Sin. B 6 (2016) 268-286. DOI:10.1016/j.apsb.2016.05.013 |
| [5] |
J.X. Gou, Y.H. Liang, L.L. Miao, et al., Acta Biomaterialia 62 (2017) 157-166. DOI:10.1016/j.actbio.2017.08.025 |
| [6] |
M. Harada, M. Ohuchi, T. Hayashi, et al., J. Controlled Release 156 (2011) 101-108. DOI:10.1016/j.jconrel.2011.06.024 |
| [7] |
L.X. Long, J. Zhao, K. Li, et al., Mater. Chem. Phys. 180 (2016) 184-194. DOI:10.1016/j.matchemphys.2016.05.062 |
| [8] |
X.T. Shuai, H. Ai, N. Nasongkla, S. Kim, et al., J. Controlled Release 98 (2004) 415-426. DOI:10.1016/j.jconrel.2004.06.003 |
| [9] |
A. Lavasanifar, J. Samuel, G.S. Kwon, Adv. Drug Deliver Rev. 54 (2002) 169-190. DOI:10.1016/S0169-409X(02)00015-7 |
| [10] |
S.B. Ruan, M.Q. Yuan, L. Zhang, et al., Biomaterials 37 (2015) 425-435. DOI:10.1016/j.biomaterials.2014.10.007 |
| [11] |
K.D. Zhao, D. Li, W.G. Xu, et al., Biomaterials 116 (2017) 82-94. DOI:10.1016/j.biomaterials.2016.11.030 |
| [12] |
M.A. Azagarsamy, P. Sokkalingam, S. Thayumanavan, J. Am. Chem. Soc. 131 (2009) 14184-14185. DOI:10.1021/ja906162u |
| [13] |
R. Banerjee, D. Dhara, Langmuir 30 (2014) 4137-4146. DOI:10.1021/la500213h |
| [14] |
J.X. Ding, F.H. Shi, C.S. Xiao, et al., Polym. Chem. 2 (2011) 2857-2864. DOI:10.1039/c1py00360g |
| [15] |
Y.Q. Zhu, J. Zhang, F.H. Meng, et al., J. Controlled Release 233 (2016) 29-38. DOI:10.1016/j.jconrel.2016.05.014 |
| [16] |
H. Zheng, L.Q. Yin, X.Q. Zhang, et al., J. Biomed. Nanotechnol. 12 (2016) 1641-1653. DOI:10.1166/jbn.2016.2279 |
| [17] |
D. Maciel, P. Figueira, S.L. Xiao, et al., Biomac. 14 (2013) 3140-3146. DOI:10.1021/bm400768m |
| [18] |
L. Dong, S.H. Xia, Y. Luo, et al., J. Controlled Release 134 (2009) 214-220. DOI:10.1016/j.jconrel.2008.11.013 |
| [19] |
J.Y. Liu, N. Li, P.L. Cai, S.L. Yang, J. Controlled Release 172 (2013) e40. |
| [20] |
C.M. Wang, J.T. Sun, Y. Luo, et al., Biotecnol. Lett. 28 (2006) 539-543. DOI:10.1007/s10529-006-0011-x |
| [21] |
Q.X. Guan, D.D. Sun, G.Y. Zhang, et al., Molecules 21 (2016) 1-16. |
| [22] |
Y.Y. Guo, W.X. He, S.F. Yang, et al., Colloids Surf. B 151 (2017) 119-127. DOI:10.1016/j.colsurfb.2016.12.012 |
| [23] |
Y.N. Zhong, W.J. Yang, H.L. Sun, et al., Biomacromolecules 14 (2013) 3723-3730. DOI:10.1021/bm401098w |
| [24] |
X.L. Jiang, L.H. Li, J. Liu, W.E. Hennink, R.X. Zhuo, Macromol Biosci. 12 (2012) 703-711. DOI:10.1002/mabi.v12.5 |
| [25] |
X.W. Wang, J.Y. Wang, Y.M. Bao, et al., RSC Adv. 4 (2014) 60064-60074. DOI:10.1039/C4RA12276C |
| [26] |
S.A. Pillai, B. Bharatiya, M. Casas, et al., Colloids Surf. B 148 (2016) 411-421. DOI:10.1016/j.colsurfb.2016.09.014 |
 2018, Vol. 29
2018, Vol. 29 


