b College of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Qingdao 266042, China
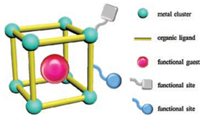
Metal-organic framework (MOF) materials have rapidly developed as a new type of porous materials with promising applications in the fields of gas storage and separation [1, 2], molecular sensing [3, 4], proton conduction [5], drug delivery [6], and catalysis [7-10]. Compared with conventional zeolites, MOF catalysts often show several advantageous features such as high surface area, uniform and tunable pores, facile functionalization and incorporation of catalytic active sites. Generally, two strategies have been used for constructing functional MOF catalysts including the pre-assembly of functional building blocks and post-synthetic modification (PSM) of MOFs with active functional sites [11]. The latter strategy is much easier and more flexible compared with the former in obtaining porous MOF catalysts with specific and controllable topologies. Functional MOF catalysts currently achieved by PSM methods consist of three established techniques (Scheme 1): (1) PSM of open metal sites via coordination bond interactions [12]; (2) PSM of organic ligands through organic synthesis [13, 14]; (3) encapsulation of functional guest molecules in the pores or cages of MOF materials [15, 16]. To date, several reviews have been published about the application of MOF as catalysts [7-10]. In this review, we aim to focus on the application of multi-functional sites (MFSs) MOF catalysts obtained by PSM methods and emphasize the cooperative or separate roles of these functional sites.
|
Download:
|
| Scheme 1. PSM methods of functional MOF catalysts. | |
2. Catalysts based on the cooperative functions of multifunctional sites via PSM method 2.1. Synergistic catalysis between guest functionalized sites
Synergistic, cooperative activation through multiple catalytic centers can often be found in enzyme biocatalysts, in which their catalytic sites can simultaneously activate multiple reacting species, thus showing exceptional efficiency and selectivity. Inspired by such biocatalysts, a lot of heterogeneous catalysts have been developed based on MOFs as supports. Bimetallic nanoparticles encapsulated in MOFs have become a type of synergistic catalysts model and examples of bimetallic NPs on MOF supports have appeared in the literatures. In 2011, Dr. Jiang and Professor Xu et al. reported a bimetallic catalyst of Au@Ag coreshell nanoparticles encapsulated in ZIF-8 by a sequential deposition-reduction method (Scheme 2) [17]. The obtained catalyst can catalyze reduction of 4-nitrophenol to 4-aminophenol by NaBH4 in water. The catalytic activity was significantly improved by the synergistic effect of bimetallic nanoparticles compared with their monometallic counterparts. In addition, Xu group also immobilized bimetallic Au-Ni NPs into the mesopores of MIL-101-Cr via a double solvent method followed by an overwhelming reduction agent [18]. The resulted catalysts were used for catalyzing hydrogen generation from ammonia borane (AB). Clearly, AuNi@- MIL-101 are more active for the hydrolysis of AB than the monometallic counterparts, suggesting synergistic effect between Au and Ni nanoparticles. Similar synergistic effect was also observed in a Ni/Pd@MIL-101 bimetallic catalyst, which was synthesized via metal-organic chemical vapor deposition (MOCVD) method [19]. This catalyst was employed in the catalytic hydrogenation reduction of 3-heptanone. In addition, bimetallic NP@MOFs as multifunctional catalysts for a cascade reaction have been reported by Professor Jiang [20]. PdAg@MIL-101 exhibits good catalytic activity and selectivity in the synthesis of secondary arylamines, in which MIL-101 affords Lewis acidity and Pd offers hydrogenation activity while Ag greatly improves selectivity to the target product. Furthermore, the bimetallic systems were also extended to Au/Co [21], Cu/Co [22], Ag/Pd [23] etc.

|
Download:
|
| Scheme 2. Schematic illustration for the preparation methods. Copied with permission [17]. Copyright 2011, American Chemical Society. | |
2.2. Synergistic catalysis between functionalized guest species and MOF frameworks
The synergistic effect can be generated through the combination of active sites on both guest species and framework of MOF materials. Duan et al. introduced the concept of using a chiral MOF framework combined with an inorganic guest catalyst into a single system capable of efficient asymmetric catalysis [24]. The authors suggested the oxygen rich Keggin-type [BW12O40]5- polyoxometalates (POM) were actually the catalytic active centers while the chiral PYIs moiety and chiral channel environment drives asymmetrical catalysis (Scheme 3). The obtained guest exchanged Ni-PYI1 by PSM to remove the cationic chiral templates to create proper hydrophilic/hydrophobic environment can catalyze the asymmetric dihydroxylation of aryl olefins with high conversion (75%-79%) and enantioselectivity (67%-95%). The work by Duan and coworkers demonstrates the ability to exploit framework nano-environments in combination with inorganic catalytically active guest species to control and promote asymmetric catalysis. Besides the role of chiral environment induction, the MOF framework can also act as another catalytically active site to participate in the catalytic reaction. For example, Martis et al. demonstrated a nanoparticles-Lewis base system by PSM immobilizing the Pd NPs into NH2-MIL-125, in which —NH2 groups act as a proton scavenger, forming —+HNH2 [25]. Meantime, —NH2 groups within the MOF framework have a positive effect on the O-H bond dissociation, and together with the Pd NPs guests are responsible for the high catalytic activity.

|
Download:
|
| Scheme 3. Synthetic procedure of Ni-PYI1, showing the guest exchange and the potential amphipathic channel for the asymmetric olefin dihydroxylation. Copied with permission [24]. Copyright 2013, American Chemical Society. | |
2.3. Synergistic catalysis between functionalized sites on MOF frameworks
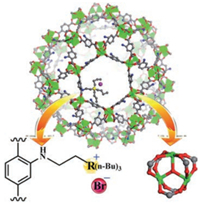
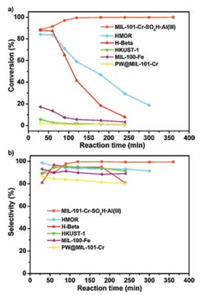
In addition to encapsulate a variety of species to MOF pores to achieve synergistic catalysis, PSM functionalized MOF frameworks show another extension for creating cooperative catalysts with multiple functional sites. In 2015, our group reported a strategy for combining CUS-based MOFs (CUS = coordinatively unsaturated metal sites) with ionic liquid (IL) functional sites to form synergistic heterogeneous catalysts with extra high activity for CO2 fixation (Fig. 1) [13]. Due to the synergetic role of dual functional sites including Lewis acid sites in the MOF framework and Br- ions in the IL functional sites, MIL-101-N(n-Bu)3Br and MIL-101-P(n-Bu)3Br exhibit high catalytic activity (yield: 99.1% and 98.6%) for the cycloaddition of CO2 and epoxide under mild and cocatalyst free conditions, which significantly outperforms other benchmark MOF catalysts. Moreover, such cooperative catalysts can be easily recovered and recycled several times without leaching and loss of activity. To develop highly effective catalysts for application in fix-bed reaction, Dr. Li and Professor Ma et al. reported a strategy to create Lewis acid@Brønsted acid MOF with high catalytic activity by PSM of Brønsted acid MOF framework with Lewis acid sites (Scheme 4) [14]. As an example, MIL-101-CrSO3H·Al(Ⅲ) demonstrates excellent catalytic performance in benzylation reaction of aromatic hydrocarbons with benzyl alcohol (BA). Importantly, the catalytic performance of MIL-101-CrSO3H·Al(Ⅲ) outperforms two benchmark zeolite catalysts (H-Beta and HMOR) (Fig. 2). The comparable experiments show the catalytic activity of MIL-101-Cr-SO3H·Al(Ⅲ) was much higher than their counterparts as well as their physical mixtures. Therefore, it is the synergy effect of their multiple functional sites that takes the responsibility of its high activity. The investigation paves the way for developing highly effective MOF-based fix-bed catalysts in the field of industrial catalysis.
|
Download:
|
| Fig. 1. The structure of the catalysts MIL-101-N(n-Bu)3Br and MIL-101-P(n-Bu)3Br (Cr: green, C: gray, O: red, N: blue, Br: amaranth, and R = N or P). Copied with permission [13]. Copyright 2015, Royal Society of Chemistry. | |

|
Download:
|
| Scheme 4. (a) Schematic presentation of the synthesis strategy for Lewis Acid@Brønsted acid MOF, in which the framework stands for the Brønsted acid functionalized MOF and the violet balls stand for Lewis acid centers; (b) Schematic presentation of the synthesis process for MIL-101-Cr-SO3H·Al(Ⅲ). Copied with permission [14]. Copyright 2015, American Chemical Society. | |
|
Download:
|
| Fig. 2. (a) Conversions of benzyl alcohol and (b) selectivities for BTMB using different catalysts as functions of time. Copied with permission [14]. Copyright 2015, American Chemical Society. | |
The PSM method has proven to be an effective strategy for the construction of multi-functional MOF catalysts. In this section, we have summarized three main strategies and highlighted some important examples. The introduction of multi-functional cooperative sites has greatly expanded the application range of MOF catalysis.
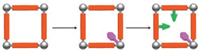
3. Catalysts based on the independent functions of multifunctional sites via PSM methodAs discussed above, MOFs provide a good model for creating multifunctional sites in inorganic building blocks, organic ligands as well as guest's moiety. If these separated functional sites can independently catalyze one or multiple steps of total reaction, then the catalyst can be used in a cascade or tandem reaction to decrease reaction time, energy and cost [26]. These independent functions can be obtained via PSM method. For example, our group has developed a strategy to create cascade catalyst via PSM of both open metal sites and organic ligands (Scheme 5) [27]. Based on this strategy, we reported the first case of organo-bifunctional MOF catalyst via PSM method and applied it in a tandem reaction. The catalyst shows two hostile functional sites in a system including strong Brønsted acid and strong Lewis base sites, while such functional sites will quench each other in a homogeneous system. But our catalyst exhibits superior catalytic performance in a cascade deacetalization-nitroaldol reaction, indicating the independent acid and base functions in a system. Such strategy provides a versatile way to modify sorts of multifunctional sites in a MOF framework. Even if the strategy uses three-step to introduce two hostile functional sites which seems time consuming. However, this strategy can allow the use of two steps to immobilize two kinds of common functional sites with high efficiency and convenience.
|
Download:
|
| Scheme 5. The generic procedure for synthesizing bifunctional MOFs by grafting the functional groups onto both organic ligands and CUSs, respectively (grey ball: metal or metal cluster; orange pillar: organic ligand; green, and lavender bar: functional groups). Copied with permission [27]. Copyright 2012, Royal Society of Chemistry. | |
The cascade catalyst can also be obtained via PSM of mixedligands MOF. For example, in 2014, Ahn et al. reported an acid-base bifunctional MOF catalyst MIL-101-NH2-SO3H [28]. They first synthesized a mixed-ligands MOF MIL-101-NO2-SO3H and then selectively reduced the —NO2 group to be —NH2 to obtain multifunctional acid-base catalysts (Scheme 6), which also show a good catalytic activity for one-pot tandem reaction. The examples of introducing two functional sites in organic ligands for constructing cascade bifunctional catalysts have been reported elsewhere [29, 30].
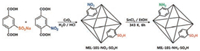
|
Download:
|
| Scheme 6. Preparation of the site-isolated acid-base bifunctional catalyst, MIL– 101–NH2–SO3H. Copied with [28] Copyright 2014, Royal Society of Chemistry. | |
Many MOF materials reported possess two different cages. If these different cages can be endowed with different functions, they will form multifunctional cages MOF materials for cascade reaction. Undoubtedly, it is hard to control the functions of these cages via pre-assembly methods and thus only limited MOF materials exhibits such dual functional cages. Recently, our group reported a controllable and versatile strategy for creating dual functional MOFs with different types of cages and pores (Fig. 3)[31]. The strategy is mainly based on two steps: first of all, introducing the functional group into MOF cages; using the window-size-controlled way to selectively encapsulate functional guest molecules into the big cages of MOF, and thus creating the dual functional MOF with two different functional cages. This strategy allows for the control of the functions of two cages to obtained different bifunctional MOFs for many different applications. Such facile PSM strategy provides a versatile way to control the functions of two cages, which is unprecedented in the synthesis of dual functional porous materials.

|
Download:
|
| Fig. 3. Multifunctional cages MOF materials for cascade reaction. Copied with permission [31]. Copyright 2016, American Chemical Society. | |
The cascade catalysis is a powerful strategy in which the reaction performed in a one pot system without separating and purifying the intermediates. The PSM method offer many opportunities for creating and improving multifunctional sites MOF catalysis for cascade reaction.
4. Conclusion and outlookPSM provides a versatile way to construct multi-functional catalysts with cooperative or cascade functions. These functions can be tuned and controlled by selecting different functional groups and functionalized ways to achieve the desired catalysts. While remarkable progress has been made in terms of the construction of multi-functional MOF catalysts via PSM, there are still many challenges that need to be addressed and greater space for improvement. First, it is difficult to determine the structure of bimetallic NP incorporated into MOFs, and the synergistic effects of bimetallic NPs on their catalytic performance are not well understood. Thus, more credible characterization technologies and theoretical calculation are in urgent need. Second, stability is a very important issue in PSM and catalysis process. Compared with some traditional inorganic porous materials, MOFs have relatively low stability. This limitation could be overcome by developing more robust new MOFs. Another challenging area of this research is the application of multi-functional MOF catalysts for cascade or tandem reaction is mainly in Lewis acid-base catalysis and hydrogenation reactions. Although some impressive results have been achieved, the catalytic reaction types should be extended in the future. Last but not least, the currently reported synergistic catalysts are generally based on dual functional sites. There are still many challenges in the design and synthesis of triple- or multifunctional MOF catalysts.
In summary, although some challenges still exist, PSM will continue to be a significant method for the construction of multifunctional MOFs because of the versatile and effective feature it possesses. In addition, the advance of MOF catalysts with multifunctional sites will pave a promising way for developing porous heterogeneous catalytic materials.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (Nos. 21371069 and 21621001).
| [1] |
B. Li, Z. Zhang, Y. Li, et al., Angew. Chem. Int. Ed. 51 (2012) 1412-1415. DOI:10.1002/anie.201105966 |
| [2] |
K. Liu, B. Li, Y. Li, et al., Chem. Commun. 50 (2014) 5031-5033. DOI:10.1039/c4cc00375f |
| [3] |
Z. Hu, B.J. Deibert, J. Li, Chem. Soc. Rev. 43 (2015) 5815-5840. |
| [4] |
D. Ma, B. Li, X. Zhou, et al., Chem. Commun. 49 (2013) 8964-8966. DOI:10.1039/c3cc44546a |
| [5] |
P. Ramaswamy, N.E. Wong, G.K.H. Shimizu, Chem. Soc. Rev. 43 (2015) 5913-5932. |
| [6] |
J.D. Rocca, D. Liu, W. Lin, Acc. Chem. Res. 44 (2011) 957-968. DOI:10.1021/ar200028a |
| [7] |
A. Dhakshinamoorthy, M. Opanasenko, J. Cejka, et al., Catal. Sci. Technol. 3 (2013) 2509-2540. DOI:10.1039/c3cy00350g |
| [8] |
L. Zeng, X. Guo, C. He, et al., ACS Catal. 6 (2016) 7935-7947. DOI:10.1021/acscatal.6b02228 |
| [9] |
J. Liu, L. Chen, H. Cui, et al., Chem. Soc. Rev. 43 (2014) 6011-6061. DOI:10.1039/C4CS00094C |
| [10] |
Q. Yang, Q. Xu, H.L. Jiang, Chem. Soc. Rev. 46 (2017) 4774-4808. DOI:10.1039/C6CS00724D |
| [11] |
J.D. Evans, C.J. Sumby, C.J. Doonan, Chem. Soc. Rev. 43 (2014) 5933-5951. DOI:10.1039/C4CS00076E |
| [12] |
M. Banerjee, S. Das, M. Yoon, et al., J. Am. Chem. Soc. 131 (2009) 7524-7525. DOI:10.1021/ja901440g |
| [13] |
D. Ma, B. Li, K. Liu, et al., J. Mater. Chem. A. 3 (2015) 23136-23142. DOI:10.1039/C5TA07026K |
| [14] |
B. Li, K. Leng, Y. Zhang, et al., J. Am. Chem. Soc. 137 (2015) 4243-4248. DOI:10.1021/jacs.5b01352 |
| [15] |
J.W. Ding, R. Wang, Chin. Chem. Lett. 27 (2016) 655-658. DOI:10.1016/j.cclet.2016.03.005 |
| [16] |
C.I. Ezugwu, B. Mousavi, M.A. Asraf, et al., J. Catalysis 344 (2016) 445-454. DOI:10.1016/j.jcat.2016.10.015 |
| [17] |
H.L. Jiang, T. Akita, T. Ishida, et al., J. Am. Chem. Soc. 133 (2011) 1304-1306. DOI:10.1021/ja1099006 |
| [18] |
Q.L. Zhu, J. Li, Q. Xu, J. Am. Chem. Soc. 135 (2013) 10210-10213. DOI:10.1021/ja403330m |
| [19] |
J. Hermannsdorfer, M. Friedrich, N. Miyajima, et al., Angew. Chem. Int. Ed. 51 (2012) 11473-11477. DOI:10.1002/anie.201205078 |
| [20] |
Y.Z. Chen, Y.X. Zhou, H. Wang, et al., ACS Catal. 5 (2015) 2062-2069. DOI:10.1021/cs501953d |
| [21] |
J. Li, Q.L. Zhu, Q. Xu, Chem. Commun. 50 (2014) 5899-5901. DOI:10.1039/c4cc00785a |
| [22] |
J. Li, Q.L. Zhu, Q. Xu, Catal. Sci. Technol. 5 (2015) 525-530. DOI:10.1039/C4CY01049C |
| [23] |
H. Dai, B. Xia, L. Wen, et al., Appl. Catal. B 165 (2015) 57-62. DOI:10.1016/j.apcatb.2014.09.065 |
| [24] |
Q. Han, C. He, M. Zhao, et al., J. Am. Chem. Soc. 135 (2013) 10186-10189. DOI:10.1021/ja401758c |
| [25] |
M. Martis, K. Mori, K. Fujiwara, et al., J. Phys. Chem. C 117 (2013) 22805-22810. DOI:10.1021/jp4069027 |
| [26] |
Y.B. Huang, J. Liang, X.S. Wang, et al., Chem. Soc. Rev. 46 (2017) 126-157. DOI:10.1039/C6CS00250A |
| [27] |
B. Li, Y. Zhang, D. Ma, et al., Chem. Commun. 48 (2012) 6151-6153. DOI:10.1039/c2cc32384b |
| [28] |
Y.R. Lee, Y.M. Chung, W.S. Ahn, RSC Adv. 4 (2014) 23064-23067. DOI:10.1039/c4ra02683g |
| [29] |
H. Liu, F.G. Xi, W. Sun, et al., Inorg. Chem. 55 (2016) 5753-5755. DOI:10.1021/acs.inorgchem.6b01057 |
| [30] |
P.V. Dau, S.M. Cohen, Inorg. Chem. 54 (2015) 3134-3138. DOI:10.1021/ic502316v |
| [31] |
B. Li, D. Ma, Y. Li, et al., Chem. Mater. 28 (2016) 4781-4786. DOI:10.1021/acs.chemmater.6b01898 |
 2018, Vol. 29
2018, Vol. 29 


