In the past few decades, the research on the design and synthesis of metal-organic frameworks (MOFs) has greatly promoted the progress of both the theory and applications of coordination chemistry. Particularly, the research on MOFs has established an extensive platform of crystalline materials with tunable chemical contents and structures, functionality, and porosity [1]. In comparison with other conventional porous materials, such as zeolites or porous carbon, MOFs have variable pore size, shape and hence a wider range of chemical applications [2-5]. MOFs have been widely used in gas storage and separation [6], ion recognition [7], catalysis [8], luminescence [9], magnetism [10] and so on. Chirality is an important feature of many materials and has the applications in chemistry, pharmacy, agriculture and medicine. The enantiomers of the same substance can show different functionalities from each other in human's bodies or the environment [11]. Recently, chemists have paid much attention to study chiral MOFs [11-17].
In general, the synthetic routes for chiral MOFs include: direct synthesis of MOFs with chiral ligands or spontaneous resolution, the introduction of chiral groups to achiral MOFs by post-synthetic modification, and the usage of chiral templates to induce chiral centers. The direct synthesis of chiral MOFs using chiral ligands or spontaneous resolution is very challenging because the products have to be crystallized in chiral space groups via self-assembly. Post-synthetic modification can be applied to either the metal centers or the ligands or even the pores. The size and shape of the pores can be well modified in this way. Using chiral or achiral template can generate chiral centers in MOFs. The selection of the template molecule is the key. In this filed, there are well-organized reviews: In 2009, Lin and his coworkers discussed the applications of charity MOFs in enantioselective catalysis [12]. In 2010, Cui and his coworkers summarized the advances in asymmetric heterogeneous catalysis and enantioselective separation [11]. In 2010, Morris and his coworkers reviewed the development of methods for the preparation of chirality [17]. In 2016, Bu and his coworkers reviewed the chiral chemistry of metal–camphorate frameworks [18]. In this review, we will mainly focus on the synthetic strategies for chiral MOFs.
2. Direct synthesisMOFs are crystalline materials, and hence to crystallize organic ligands coordinated with metal ions into a chiral space group is very challenging due to the assembly of all the components into the requested symmetries of the chiral space group. There are two main methods to obtain chiral MOFs directly as follows.
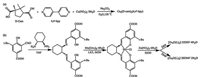
2.1. Using enantiopure ligands for chiral MOFsThe key of using chiral ligands to construct chiral MOFs is the selection and synthesis of the chiral ligands. According to the origin of the chirality, those ligands can be divided into two categories: central chiral ligands and axial chiral ligands. For example, in 2000, Kim and his coworkers used (4S, 5S)-2, 2- dimethyl-5-(pyridin-4-ylcarbamoyl)-1, 3-dioxolane-4-carboxylic acid (HDPCDC) to construct D-POST-1, [Zn3(μ3-O)(DPCDC)6]·2H3O·12H2O, for the catalytic reactions of the transesterification. [18]. Another central chiral ligand, camphor acid, has been well studied. Bu and his coworkers have summarized in detail about the MOFs with camphor acids [19]. In 2007, Bu used 4, 4'-bipyridine (4, 4'-bpy) and D-(+)-camphoric acid (D-Cam) to construct [Cu2(D-Cam)2(4, 4'-bpy)] (Fig. 1a) [20]. In addition, hydroxy acid (such as lactic acid, malic acid and tartaric acid), nucleotide, alkaloid and biotin are commonly used to construct chiral MOFs.
|
Download:
|
| Fig. 1. Using (a) central chiral ligand (b) axial chiral ligand for chiral MOFs. | |
The construction of chiral MOFs using amino acids is also a useful way. In 2014, Rosseinsky and his coworkers used the natural dipeptide carnosine (β-alanyl-L-histidine) to construct [Zn2(β-alanyl-L-histidine)2]·6H2O, which is an analogue of ZIFs where the imidazole ring of the histidine side chain affords the chemical stability of the framework in water [21]. In 2015, Wang and his coworkers used D-histidine, which has very similar structure of 2- methylimidazole, to modify the cages of ZIF-8 to be chiral, which can be used to catalyze asymmetric synthesis [22]. In 2016, UHM- 25, [Cu2DDBBA(H2O)2] (H4DDBBA = 1, 1-di-(3', 5'-dicarboxybiphenyl)-N-tert-butyloxycarbonyl-alaninol) is synthesized with a large chiral ligand [23]. By using the carboxyl group of the chiral amino acid and the lithium metal of the alkyl lithium reagent, the chiral center of the bromide is formed. This series of chiral MOFs have a catalytic effect on the condensation of acetaldehyde.
In 2015, Zaworotko and his coworkers reported a chiral MOF, [Co2(mandelate)2(4, 4'-bipyridine)3](NO3)2·guest (CMOM-1), by which they revealed that relatively high ee values can be obtained through confined space based on the homochirality of the molecular building blocks via van der Waals forces, hydrogenbonding interactions, and π–π stacking interactions. [24]. Recently, Zaworotko and his coworkers changed the shape and size of the hole to construct a dynamic chiral MOF, CMOM-3S, based on mandelic acid. This is the first MOF that can serve as both a generalpurpose chiral crystalline sponge and a chiral stationary phase for gas chromatography. Simple synthetic routes and stable structures make it great potential applications in chiral purification and enantiomer identification [25].
On the other hand, the study of MOFs with axial chiral ligands is also interesting. Lin and his coworkers synthesized a series of chiral binaphthyl derivatives with a variety of substituent groups, such as carboxylic acid (Fig. 1b) [26-30], pyridine [31, 32] and phosphoric acid [33, 34]. The frameworks with transition metal ions and the catalytic effect on asymmetric synthesis are investigated. The length of the ligand and pore size are well controlled by the number of benzene rings of the ligands. In 2009, Cui and his coworkers synthesized a relatively large ligand with C3 symmetry, namely tris((4-carboxyphenyl)aryl)borane (H3L1), to construct a series of chiral MOFs, [M2(L1)(OH)(MeOH)]·3H2O [M = Co, Mn, Ni, Cu, Zn and Cd] [35]. Recently, Cui and his coworkers used N, N'-bis(3-tert-butyl-5-(carboxyl)salicylide (L2) to construct multivariate MOF of [Zn4O(L2-Cu)3] [36]. Similar to the naphthalene structure, the MOFs get chirality by its axial of the ligand. In 2016, Yaghi and his coworkers used Al8(μ-OH)8(HCOO)4(-COO)12 and 1, 3, 5-benzenetribenzoate (H3BTB) to construct a chiral MOF-520, Al8(μ-OH)8(HCOO)4(BTB)4 [37]. They used the open metal sites to embed eleven achiral molecules and five chiral molecules by the coordination with hydroxyl or carboxyl groups.
In general, using enantiopure ligands to construct chiral MOFs directly is very challenging in synthesis. It is important to synthesis new chiral ligands to construct new chiral MOFs with chiral nano space for targeted applications.
2.2. Spontaneous resolution of chiral MOFs from achiral componentsThe achiral species could also form chiral products from spontaneous resolution by forming the helical structures induced by entanglement of molecular chains. Unlike the direct method, the self-assembly of the achiral species and entanglement of molecular chains is unpredictable, which is much challenging in the design and synthesis. However, the formation of helical chains for chiral construction has the advantage of using easily available achiral ligands [38]. Aoyama and his coworkers reported the first homochiral MOF Cd(L3)(NO3)2(H2O)(CH3CH2OH) based on achiral 5-(9-anthracenyl)pyrimidine (L3) in 1999 [39].
In 2007, Zheng et al. reported the reaction of succinic acid and 4, 4'-bipyridine with Cu2+ ions to form a helical chiral MOF [Cu (succinate)(4, 4'-bipyridine)]·4H2O [40]. They proposed a possible mechanism for the formation of the chiral product in comparison with a coin flip: If a coin is flipped enough times, the probability of positive and negative is equal. However, when the flipped number is not enough, even in extreme cases only once, the situation is different. The probability of one side is much higher than the probability of the other side. For the formation of the chiral MOF, when the first coordination polymer nucleates, it has an induction effect for the next, so that the whole system is inclined to one direction. The optical property of the final product suggested pure chiral nature of the MOF. This explained why using achiral ligands to form helical chains yields chiral products rather than racemic frameworks.
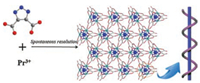
In 2005, Qiu and his coworkers constructed [Co(PDC)(H2O)2]· H2O based on pyridine-2, 5-dicarboxylate (H2PDC) and Cu2+ ions. The ligands as chiral inductions played a key role in the formation of helical chains [41]. In 2014, Cheng and his coworkers used 1H- [1, 2, 3]-triazole-4, 5-dicarboxylic acid (H3TDA) to construct isostructural chiral [Pr(TDA)(H2O)]·2.5H2O and [Nd(TDA)(H2O)]· 2.5H2O (Fig. 2) [42].
|
Download:
|
| Fig. 2. Spontaneous resolution for chiral MOFs from achiral components. | |
The synthetic strategy of direct method for the construction of chiral MOFs is simple. The challenges using this strategy are mainly from that chiral reagents are often expensive and crystallizing them with metal ions in low-symmetry chiral space groups is much complicated than the achiral space group and the product is not enantiopure. Factors influencing the crystallization process should be studied for the rational design and synthesis of chiral MOFs.
3. Indirect methodThere are two main methods to obtain chiral MOFs via indirect method: Post-synthetic method and chiral induction.
3.1. Post-synthetic methodSince 1990, post-synthetic modification has been proposed by Hoskins and Robson [43], and is introduced into the study of MOFs by Wang and Cohen [44]. In general, both metal and organic components can be functionalized using the post-synthetic modification without affecting the skeleton, which is an excellent tool for functional modification of the skeleton of MOFs [45]. This method has been introduced into the construction of chiral MOFs, and has shown positive effect on applications such as asymmetric catalysis by varying the size, shape and chemical environment of the pore.
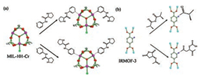
Some MOFs can have open metal sites when they are activated, suggesting that they have potential coordination abilities on metal site. In 2009, Kim and his coworkers studied the specific structural features of MIL-101 (Fig. 3a) [46]. Two different chiral ligands, (S)-N-(pyridin-3-yl)-pyrrolidine-2-carboxamide) (L4) and (S)-N-(pyridin-4-yl)-pyrrolidine-2-carboxamide) (L5), are introduced into MIL-101 by the coordination between L4/L5 and open metal sites, to generate two chiral MOFs, [Cr3O(L4)1.8(H2O)0.2F(bdc)3]·0.15 (H2bdc)·H2O and [Cr3O(L5)1.75(H2O)0.25F(bdc)3]·0.15(H2bdc)·H2O (H2bdc = 1, 4-benzenedicarboxylic acid), which have the applications in the catalytic asymmetric aldol condensation with the yield > 90% and 80% ee.
|
Download:
|
| Fig. 3. Post-synthetic method to (a) the metal center and (b) the ligand. | |
For the post-modification to the ligands for chiral MOFs, the key is that the organic ligands should have functional groups to react with chiral reagents. This method is usually implemented by substituent groups such as -Br, -NH2, -CH3, etc. [44]. For example, in 2009, Cohen and his coworkers modified the amino groups on IRMOF-3 by using two kinds of chiral anhydrides, and two chiral MOFs, IRMOF-3-(S)-AM3Me and IRMOF-3-(S)-AM-SucAcO, were obtained (Fig. 3b) [47]. In 2010, Yaghi and his coworkers performed ring opening reaction with ring amino group for chiral derivatization of IRMOF-3 to irreversible binding of a range of functionalities that are otherwise difficult to access in MOFs, to give [Zn4O(C11H12N2O4)3(C3H7N)0.24] [48]. In 2015, Farrusseng and his coworkers reported the reaction of Al-MIL-101-NH2/In-MIL- 68-NH2/Zr-UIO-66-NH2 with proline and other chiral amino acids and their derivatives to catalyze aldol condensation [49]. In addition to substituent groups, double bonds are also active groups to be post-modified. In 2009, Bauer and his coworkers synthesized a chiral MOF, [Zn4O(trans-SDC)3-Br2] (SDC = 4, 4'-stilbene dicarboxylate), by the electrophilic addition of Br2 to the double bonds [50]. In 2016, Forgan and his coworkers used the N-bromosuccinimide and extended UIO-66 to synthesize a chiral MOF of [Zr6O4(OH)4(meso-SDC-Br-OH)6] [51].
The chiral ligands may still have un-coordinated functional groups, which can be used for further modification. Lin and his coworkers used CdCl2 and 6, 6'-dichloro-2, 2'-dihydroxy-1, 1'- binaphthyl-4, 4'-bipyridine (L6) to construct a chiral MOF, [Cd3Cl6(L6)3]·4DMF·6MeOH·3H2O. The introduction of Ti(OiPr)4 into this MOF catalyzes the Ti(Ⅳ)-catalyzed ZnEt2 additions to aromatic aldehydes with > 99% yield and > 90% ee [31].
3.2. Chiral inductionChiral MOFs can be synthesized via appropriate chiral templates. Rosseinsky and his coworkers used chiral 1, 2-propanediol (1, 2-pd) as inducer to construct two chiral MOFs, [Ni3(btc)2(py)6(1, 2-pd)3]·11(1, 2-pd)·8H2O [52] and [Ni3(btc)2(3- pic)6(1, 2-pd)3]·9(1, 2-pd)·11H2O [53] (H3btc = trimesic acid, py = pyridine, 3-pic = 3-picoline) in 2000. In 2007, Morris and his coworkers used the ionic liquid 1-butyl-3-methylimidazolium-Laspartate (BMIm) as template to obtain (BMIm)2[Ni(TMAH)2(H2O)2] (H3TMA = trimesate acid) [54]. Without the ionic liquid, no chiral MOF was obtained. In 2008, Bu and his coworkers used (-)-cinchonidine and (+)-cinchonine as template to get chiral (Me2NH2)[In(thb)2]·xDMF (H2thb = thiophene-2, 5-dicarboxylic acid). In comparison with the previous work, Bu and his coworkers do not use chiral solvents. They firstly used chiral solutes as templates to induce the chirality to the MOFs [55].
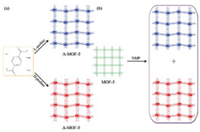
One interesting work is the construction of chiral MOF-5 by Zaworotko and his coworkers in 2015. In addition to the use of L-proline as chiral template to induce skeleton distortion of MOF-5, they also used the achiral N-methylpyrrolidone (NMP) to make MOF-5 distorted and obtained racemic products (Fig. 4) [56]. Coudert et al. have discussed this unusual phenomenon based on calculation. This phenomenon is due to the interaction of NMP with the benzene and the metal cluster on this MOF, which causes MOF-5 to be distorted, and the spatial chirality [57]. This provides a new method to construct chiral MOFs using common achiral MOF and common achiral solvent as the inducer.
|
Download:
|
| Fig. 4. Chiral induction by (a) chiral template proline and (b) achiral template N-methylpyrrolidone. Reprinted with permission [56]. Copyright 2015, American Chemical Society. | |
The mechanism of using templating agents for the synthesis of chiral MOFs is complicated. The choice of template and the mechanism of its action also need to be further understood for future studies.
4. ConclusionTwo main methods for the construction of chiral MOFs at this stage were summarized. Although successful examples have been reported, the surface area of most chiral MOFs constructed by direct synthesis is not high except for specific chiral MOFs with axis chiral ligands.
The advantage of constructing chiral MOFs by post-synthetic modification is that post-synthetic modification can develop chiral MOFs with improved gas sorption, catalytic activity, bioactivity, and physical properties by rational design of the structures and functions. Moreover, a relatively high surface area can be maintained. It is the key to have a specific functional inorganic or organic group for post-synthetic modification. Based on the design of a specific pore shape, molecular recognition of a particular pore shape and chemical environment for enhanced enantioselectivity can be achieved. The catalytic effect of asymmetric synthesis can also be obtained via this strategy.
Chiral induction is another useful way for chiral MOFs. First, the selection of suitable inducer for chiral induction can be made for known MOFs rather than rigidly adhering to the MOFs with specific functional groups. Secondly, the skeletons of the MOFs are almost not changed using this strategy. Moreover, the properties and functions of the new chiral MOFs can be regulated by the initial MOFs. The main issue of this strategy is that the selection of the template is not generally applicable, which makes it very challenging in the future studies of chiral MOFs.
In addition to chiral MOFs, covalent organic frameworks (COFs) represent an alternative family of crystalline framework materials with a wide scope of structures and potential applications, such as in asymmetric catalysts [58-61]. COFs have robust architectures endowed by the strong bonding between the building units, and hence resulted in high porosity and stability, which have allowed chemical reactions to be carried out with these frameworks [62]. But for chiral COFs, the chiral building units are usually costly and the synthesis is of great challenging, which are the main issues in the study of chiral COFs.
The study on the construction of chiral MOFs will still be one of the hot topics in MOF chemistry because of their promising applications in chiral separation, chiral recognition and asymmetric catalytic synthesis. Chiral MOFs not only contain the unique advantages of its pore structures, but also have significant contributions to the chiral world.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21622105 and 91422302), the Natural Science Foundation of Tianjin (No. 15JCYBJC47000) and the Fundamental Research Funds for the Central Universities.
| [1] |
H. Furukawa, K.E. Cordova, M. O'Keeffe, O.M. Yaghi, Science 341 (2013) 974-975. |
| [2] |
H.C. Zhou, J.R. Long, O.M. Yaghi, Chem. Rev. 112 (2012) 673-674. DOI:10.1021/cr300014x |
| [3] |
J.R. Long, O.M. Yaghi, Chem. Soc. Rev. 38 (2009) 1213-1214. DOI:10.1039/b903811f |
| [4] |
H.C. Zhou, S. Kitagawa, Chem. Soc. Rev. 43 (2014) 5415-5418. DOI:10.1039/C4CS90059F |
| [5] |
G. Maurin, C. Serre, A. Cooper, G. Fe'rey, Chem. Soc. Rev. 46 (2017) 3104-3107. DOI:10.1039/C7CS90049J |
| [6] |
J.R. Li, R.J. Kupplera, H.C. Zhou, Chem. Soc. Rev. 38 (2009) 1477-1504. DOI:10.1039/b802426j |
| [7] |
W. Lu, Z. Wei, Z.Y. Gu, et al., Chem. Soc. Rev. 43 (2014) 5561-5593. DOI:10.1039/C4CS00003J |
| [8] |
L. Zhu, X.Q. Liu, H.L. Jiang, L.B. Sun, Chem. Rev. 117 (2017) 8129-8176. DOI:10.1021/acs.chemrev.7b00091 |
| [9] |
X.Y. Ren, L.H. Lu, Chin. Chem. Lett. 26 (2015) 1439-1445. DOI:10.1016/j.cclet.2015.10.014 |
| [10] |
K. Liu, X.J. Zhang, X.X. Meng, et al., Chem. Soc. Rev. 45 (2016) 2423-2439. DOI:10.1039/C5CS00770D |
| [11] |
Y. Liu, W. Xuan, Y. Cui, Adv. Mater. 22 (2010) 4112-4135. DOI:10.1002/adma.v22:37 |
| [12] |
L. Ma, C. Abney, W. Lin, Chem. Soc. Rev. 38 (2009) 1248-1256. DOI:10.1039/b807083k |
| [13] |
J. Lee, O.K. Farha, J. Roberts, K.A. Scheidt, J.T. Hupp, Chem. Soc. Rev. 38 (2009) 1450-1459. DOI:10.1039/b807080f |
| [14] |
J.M. Fraile, J.I. García, J.A. Mayoral, Chem. Rev. 109 (2009) 360-417. DOI:10.1021/cr800363y |
| [15] |
R.J. Kuppler, D.J. Timmons, Q. Fang, et al., Coord. Chem. Rev. 253 (2009) 3042-3066. DOI:10.1016/j.ccr.2009.05.019 |
| [16] |
M. Heitbaum, F. Glorius, I. Escher, Angew Chem. Int. Ed. 45 (2006) 4732-4762. DOI:10.1002/(ISSN)1521-3773 |
| [17] |
R.E. Morris, X.H. Bu, Nat. Chem. 2 (2010) 353-361. DOI:10.1038/nchem.628 |
| [18] |
J.S. Seo, D. Whang, H. Lee, et al., Nature 404 (2000) 982-986. DOI:10.1038/35010088 |
| [19] |
Z. Gu, C. Zhan, J. Zhang, X.H. Bu, Chem. Soc. Rev. 45 (2016) 3122-3144. DOI:10.1039/C6CS00051G |
| [20] |
J. Zhang, Y.G. Yao, X. Bu, Chem. Mater. 19 (2007) 5083-5089. DOI:10.1021/cm071689a |
| [21] |
A.P. Katsoulidis, K.S. Park, D. Antypov, et al., Angew. Chem. Int. Ed. 53 (2014) 193-198. DOI:10.1002/anie.v53.1 |
| [22] |
J. Zhao, H. Li, Y. Han, et al., J. Mater Chem. A 3 (2015) 12145-12148. DOI:10.1039/C5TA00998G |
| [23] |
M. Sartor, T. Stein, F. Hoffmann, M. Froba, Chem. Mater. 28 (2016) 519-528. DOI:10.1021/acs.chemmater.5b03723 |
| [24] |
S.Y. Zhang, L. Wojtas, M.J. Zaworotko, J. Am. Chem. Soc. 137 (2015) 12045-12049. DOI:10.1021/jacs.5b06760 |
| [25] |
S.Y. Zhang, C.X. Yang, W. Shi, et al., Chem 3 (2017) 281-289. DOI:10.1016/j.chempr.2017.07.004 |
| [26] |
L. Ma, C. Wu, M.M. Wanderley, W. Lin, Angew. Chem. Int. Ed. 49 (2010) 8244-8248. DOI:10.1002/anie.v49:44 |
| [27] |
L. Ma, J.M. Falkowski, C. Abney, W. Lin, Nature Chem. 2 (2010) 838-846. DOI:10.1038/nchem.738 |
| [28] |
M. Zheng, Y. Liu, C. Wang, S. Liu, W. Lin, Chem. Sci. 3 (2012) 2623-2627. DOI:10.1039/c2sc20379k |
| [29] |
L. Ma, D.J. Mihalcik, W. Lin, J. Am, Chem. Soc. 131 (2009) 4610-4612. DOI:10.1021/ja809590n |
| [30] |
M.M. Wanderley, C. Wang, C. Wu, W. Lin, J. Am. Chem. Soc. 134 (2012) 9050-9053. DOI:10.1021/ja302110d |
| [31] |
C. Wu, A. Hu, L. Zhang, W. Lin, J. Am. Chem. Soc. 127 (2005) 8940-8941. DOI:10.1021/ja052431t |
| [32] |
G. Yuan, C. Zhu, Y. Liu, W. Xuan, Y. Cui, J. Am. Chem. Soc. 131 (2009) 10452-10460. DOI:10.1021/ja901154p |
| [33] |
A. Hu, H.L. Ngo, W. Lin, J. Am. Chem. Soc. 125 (2003) 11490-11491. DOI:10.1021/ja0348344 |
| [34] |
H.L. Ngo, W. Lin, J. Am. Chem. Soc. 124 (2002) 14298-14299. DOI:10.1021/ja027892i |
| [35] |
Y. Liu, W. Xuan, H. Zhang, Y. Cui, Inorg. Chem. 48 (2009) 10018-10023. DOI:10.1021/ic9002675 |
| [36] |
Q.C. Xia, Z.J. Li, C.X. Tan, et al., J. Am. Chem. Soc. 139 (2017) 8259-8266. DOI:10.1021/jacs.7b03113 |
| [37] |
S. Lee, E.A. Kapustin, O.M. Yaghi, Science 353 (2016) 808-811. DOI:10.1126/science.aaf9135 |
| [38] |
L. Ma, C. Abney, W. Lin, Chem. Soc. Rev. 38 (2009) 1248-1256. DOI:10.1039/b807083k |
| [39] |
T. Ezuhara, K. Endo, Y. Aoyama, J. Am. Chem. Soc. 121 (1999) 3279-3283. DOI:10.1021/ja9819918 |
| [40] |
S. Wu, Y. Wu, Q. Kang, et al., Angew. Chem. Int. Ed. 46 (2007) 8475-8479. DOI:10.1002/(ISSN)1521-3773 |
| [41] |
G. Tian, G. Zhu, X. Yang, et al., Chem. Commun. 11 (2005) 1396-1398. |
| [42] |
X.P. Zhang, N. Xu, S.Y. Zhang, X.Q. Zhao, P. Cheng, RSC Adv. 4 (2014) 40643-40650. DOI:10.1039/C4RA06629D |
| [43] |
B.F. Hoskins, R. Robson, J. Am. Chem. Soc. 112 (1990) 1546-1554. DOI:10.1021/ja00160a038 |
| [44] |
K.K. Tanabe, S.M. Cohen, Chem. Soc. Rev. 40 (2011) 498-519. DOI:10.1039/C0CS00031K |
| [45] |
Z. Wang, S.M. Cohen, J. Am. Chem. Soc. 129 (2007) 12368-12369. DOI:10.1021/ja074366o |
| [46] |
M. Banerjee, S. Das, M. Yoon, et al., J. Am. Chem. Soc. 131 (2009) 7524-7525. DOI:10.1021/ja901440g |
| [47] |
S.J. Garibay, Z. Wang, K.K. Tanabe, S.M. Cohen, Inorg. Chem. 48 (2009) 7341-7349. DOI:10.1021/ic900796n |
| [48] |
D. Britt, C. Lee, F.J. Uribe-Romo, H. Furukawa, O.M. Yaghi, Inorg. Chem. 49 (2010) 6387-6389. DOI:10.1021/ic100652x |
| [49] |
J. Bonnefoy, A. Legrand, E.A. Quadrelli, J. Canivet, D. Farrusseng, J. Am. Chem. Soc. 137 (2015) 9409-9416. DOI:10.1021/jacs.5b05327 |
| [50] |
S.C. Jones, C.A. Bauer, J. Am. Chem. Soc. 131 (2009) 12516-12517. DOI:10.1021/ja900893p |
| [51] |
R.J. Marshall, S.L. Griffin, C. Wilson, R.S. Forgan, Chem. Eur. J. 22 (2016) 4870-4877. DOI:10.1002/chem.201505185 |
| [52] |
C.J. Kepert, T.J. Prior, M.J. Rosseinsky, J. Am. Chem. Soc. 122 (2000) 5158-5168. DOI:10.1021/ja993814s |
| [53] |
D. Bradshaw, T.J. Prior, E.J. Cussen, J.B. Claridge, M.J. Rosseinsky, J. Am. Chem. Soc. 126 (2004) 6106-6114. DOI:10.1021/ja0316420 |
| [54] |
Z. Lin, A.M.Z. Slawin, R.E. Morris, J. Am. Chem. Soc. 129 (2007) 4880-4881. DOI:10.1021/ja070671y |
| [55] |
J. Zhang, S. Chen, T. Wu, P. Feng, X. Bu, J. Am. Chem. Soc. 130 (2008) 12882-12883. DOI:10.1021/ja805272j |
| [56] |
S.Y. Zhang, D. Li, D. Guo, et al., J. Am. Chem. Soc. 137 (2015) 15406-15409. DOI:10.1021/jacs.5b11150 |
| [57] |
J.D. Evans, F. Coudert, J. Am. Chem. Soc. 138 (2016) 6131-6134. DOI:10.1021/jacs.6b02781 |
| [58] |
J. Zhang, X. Han, X. Wu, Y. Liu, Y. Cui, J. Am. Chem. Soc. 139 (2017) 8277-8285. DOI:10.1021/jacs.7b03352 |
| [59] |
X. Han, Q. Xia, J. Huang, et al., J. Am. Chem. Soc. 139 (2017) 8693-8697. DOI:10.1021/jacs.7b04008 |
| [60] |
X. Wang, et al., J. Am. Chem. Soc. 138 (2016) 12332-12335. DOI:10.1021/jacs.6b07714 |
| [61] |
G. Liu, J. Sheng, Y. Zhao, Sci. China Chem. 60 (2017) 1015-1022. |
| [62] |
C.S. Diercks, O.M. Yaghi, Science 355 (2017) 923-924. |
 2018, Vol. 29
2018, Vol. 29 


