b Department of Chemistry, University of Virginia, Charlottesville, VA 22904, United States;
c Chemical Sciences Division, Oak Ridge National Laboratory, Oak Ridge 37830, United States
Semiconductor-based photocatalysis has been considered as a "green" route for the degradation of organic pollutants and the production of hydrogen fuel, and is playing an increasingly important role in our society's transition to sustainable energy and environmentally benign future [1-3]. Most of the widely used semiconductor materials, such as TiO2 and ZnO, are only photocatalytically active under the UV-light irradiation [4, 5]. As UV-light constitutes no more than 4% of the solar energy, these pristine semiconductor photocatalysts tend to be of low efficiency [6-10]. It is highly desirable to develop new and efficient semiconductor photocatalysts that can directly harness and utilize visible light.
BiOBr (bismuth oxybromide) is a tetragonal matlockite structured Ⅴ-Ⅵ-Ⅶ ternary compound that has a unique layered structure with [Bi2O2] slabs interleaved by double slabs of halogen atoms [11, 12]. It presents a suitable band gap (about 2.75 eV) and an outstanding chemical stability, making it a promising candidate visible light driven photocatalysis [13-15]. However, BiOBr suffers from the slow charge transfer rate and the high recombination probability of the photogenerated electron-hole pairs, which limit its photocatalytic activity in the practical applications. In order to improve the photocatalytic performance, different methods have been used to modify BiOBr, including generating oxygen vacancy or defect, building ultrathin or even monolayer structures [16], and constructing Bi-rich composites [17-19] and heterostructures [20, 21], and doping metals [22, 23] etc. These structural modification approaches have been proved to be effective in tailoring the band-edge position and facilitating charge separation, consequently increasing the photocatalytic activity of BiOBr.
In this communication, we report a facile approach to synthesize carbon quantum dots (CQDs)/BiOBr composites where the incorporation of CQDs can greatly improve BiOBr's photocatalytic efficiency under visible light. CQDs are an emerging class of carbon-related materials with many appealing properties such as low-cost, earth abundance, high biocompatibility and ecofriendliness [24]. Previous studies have shown that CQDs, after compositing with the semiconductor materials, can serve as an electron reservoir as well as a photo-induced electron transporter to promote the charge carrier migration and separation within semiconductors. Therefore, bycoupling semiconductors, such as g-C3N4 [25], TiO2 [26], Fe2O3 [27], Ag3PO4 [28], Cu2O [29] and BiVO4 [30], with CQDs, the composites have shown significantly enhanced photocatalytic performance for organic pollutant removal. Meanwhile, the CQDs have been reported to exhibit up-conversion photoluminescence (PL) properties, which might provide another way to maximize solar energy utilization efficiency [31]. Considering these unique properties of BiOBr and CQDs, we anticipate the construction of CQDs/BiOBr composites could be a promising pathway to highly efficient photocatalysts. Correspondingly, we develop a hydrothermal synthesis to the CQDs/BiOBr composites with well controlled CQDs concentrations.
The resultant CQDs/BiOBr composites present a remarkably enhanced photocatalytic activitycompared with the pristine BiOBr for the degradation of antibiotic tetracycline (TC), endocrine disrupter bisphenol A (BPA) and rhodamine B (RhB) under visible light irradiation. By systematically characterizing the obtained CQDs/BiOBr composites, the key role of CQDs in photocatalysis enhancement is carefully discussed. This investigation provides a new and generalized strategy for the design and fabrication of CQDs-promoted photocatalysts for environmental applications and beyond.
All chemicals used in this work were of analytical grade, and were used without further purification. The ionic liquid [C16mim]Br (1-hexadecyl-3-methylimidazolium bromide) (99%) was purchased from Shanghai Chengjie Chemical Co., Ltd. The CQDs solution was prepared using the method reported by Yang et al. [24].
In a typical synthesis of CQDs/BiOBr composite, 1 mmol ionic liquid [C16mim]Br was mixed into a certain amount of CQDs stock solution (0.5mL, 1mL, 2mL, or 5mL, weight concentration: 5mg/mL), followed by dilution with 20mL deionized water. 1 mmol of Bi(NO3)3·5H2O was then dissolved into the mixture. The pH of the solution was adjusted to 1 by using nitric acid. After that, the mixture was stirred in air at room temperature for 0.5h, and then transferred into 25mL Teflon-lined autoclave, which was sealed and heated at 140 ℃ for 24h under autogenous pressure. The product was collected by centrifugation, washed with distilled waterand ethanol for threetimes, and dried under vacuum at 50 ℃ for 24h. As-prepared photocatalysts were named as 0.8wt% CQDs/BiOBr (0.5mL CQDs solution), 1.6wt% CQDs/BiOBr (1mL CQDs solution), 3.1wt% CQDs/BiOBr (2mL CQDs solution), 7.5wt% CQDs/BiOBr (5mL CQDs solution), respectively.
X-ray powder diffraction (XRD) analysis was carried out on a Bruker D8 diffractometer with high-intensity Cu-Kα radiation (λ=1.54Å). FT-IR spectrum was collected on a Nicolet FT-IR spectrophotometer (Nexus 470, Thermo Electron Corporation) using KBr disks. Raman spectra were collected on an HR 800 Raman spectroscope (J Y, France) equipped with a synapse CCD detector and a confocal Olympus microscope. The field-emission scanning electron microscopy (FE-SEM) measurements were carried out under a field-emission scanning electron microscope (JEOL JWSM-7001F) equipped with an energy-dispersive X-ray spectroscope (EDS), operated at an acceleration voltage of 10kV. Transmission electron microscopy (TEM) images were taken on a JEOL-JEM-2010 (JEOL, Japan) operating at 200kV. UV–vis diffuse reflectance spectrum was recorded on an UV-2450 spectrophotometer (Shimadzu Corporation, Japan) using BaSO4 as the reference. The PL spectra of the photocatalysts were collected using a VarianCaryEclipse spectrometer (U.S.A.).Photocurrentand electrochemical impedance spectroscopy were collected on an electrochemical potentiostat (CHI 660B Chenhua Instrument Company, Shanghai, China).
To comparethe photocatalytic activity of BiOBr and CQDs/BiOBr composites, a series of photodegradation experiments were carried out under the irradiation of visible-light, using a 300W Xe lamp with a 400nm cutoff filter as the light source, and TC, BPA, and RhB as model pollutants. Experiments were carried out in a Pyrex photocatalytic reactor with a circulating water system to prevent thermal catalytic effects. In a typical photocatalytic experiment, 0.03g of sample was dispersed into 100mL of TC (20mg/L), 0.05g of sample was dispersed into 100mL of BPA (10mg/L), or 0.02g of sample was dispersed into 100mL of RhB (10mg/L) solution, respectively. Prior to irradiation, the suspensions were magnetically stirred for 30min in the dark to achieve a saturated RhB adsorption onto the photocatalyst surface. During the reaction, 3mL suspension was collected at the interval of 0.5h. The suspension was centrifuged (13000rpm, 3min) to remove the photocatalyst particles and the remaining pollutant (TC, BPA or RhB) concentration was analyzed with a UV–vis spectrophotometer (UV-2450, Shimadzu) at the absorption wavelengths of 356nm (for TC) or 553nm (for RhB). The removal of BPA was monitored by an Agilent high performance liquid chromatography (HPLC) system with a TC-C (18) column and a variable wavelength detector (VWD), with the wavelength setting at 230nm. A mixture of methanol and purewater (volume ratiowas 3:1) was used as the mobile phase, with a flow rate of 1.0 mL/min.
To investigate the change of photogenerated electrons before and after the introduction of CQDs in the BiOBr, the photocurrents were measured with an electrochemical analyzer using a standard three-electrode system. To prepare the working electrode, 2mg of the as-prepared BiOBr or CQDs/BiOBr was dispersed into a mixture of 0.2mL of ethanol and 0.2mL of EG toform a uniform suspension, which was then dip-coatedonto a 0.5 ×1 cm2 ITO glass electrode.A platinum wire and a saturated Ag/AgCl electrode were employed as the counter and reference electrodes, respectively. A 500W Xe arc lamp was utilized as the photosource. A phosphate buffered saline (0.1mol/L, pH 7.0) was used as the electrolyte for photocurrent investigation. Electrochemical impedance spectroscopy (EIS) measurement was performed using a CHI 660B workstation. The Nyquist plots were recorded from 100MHz to 100kHz in a 0.1mol/L KCl solution containing 5mmol/L Fe(CN)63-/(Fe(CN)64-. All the electrochemical measurements were performed under sunless conditions.
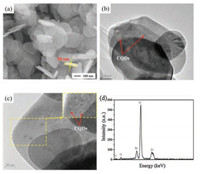
In our previous work, we have demonstrated the synthesis of CQDs/BiOBr composite photocatalysts by using the freeze-dried CQD powders [32]. The CQDs synthesized using citric acid via a hydrothermal process possessed carboxyl groups, which interacted with the [C16mim]+ to form micelles in the ionic liquid solution. These micelles served as the soft template to form BiOBr nanosheets. However, we noticed that the freeze-drying approach may deactivate the surface functional groups of CQDs, leading to a less-effective interaction between CQD and BiOBr. As a result, while most of the CQDs were dispersed on the BiOBr's surface, we observed a small portion of free CQDs that were not attached tothe BiOBr nanosheets. To maintain the surface reactivity of CQDs and improve the CQD - BiOBr interaction, the CQDs stock solution was directly employed without freeze-drying in the present study. The enhanced interaction between CQD and BiOBr and the improved dispersion of CQDs are well-identified by the morphology investigation. As shown in Fig. 1a, the 3.1wt% CQDs/BiOBr is consistedof an arrayof nanosheets and each sheet has a lateral size of about 150nm, and a thickness of ca. 20nm. In order to further visualize the details of CQDs, TEM has been employed and the recorded results are shown in Figs. 1b and c. The nanosheet structure is further confirmed in Fig. 1b, with no unattached free CQDs in all the investigated area. The enlarged TEM image (Fig. 1c) shows that all CQDs are of an average diameter of 5nm and uniformly anchored onto the surface of BiOBr nanosheets. These results confirm that by directlyemploying CQDs stock solutionwill effectively avoid the surface deactivation that occurred on the freeze-dried CQDs powders, leading to a better interaction between CQDs and BiOBr and the dispersion of CQDs in the composites. The EDS pattern (Fig. 1d) indicated the existence of C, O, Br, and Bi in the 3.1 wt% CQDs/BiOBr composite. While Bi, O and Br are attributed to the BiOBr, C is mainly from the CQDs. Based on the ratio of C, the content of CQDs is determined to be about 2.6%, which is close to the theoretical value in 3.1 wt% CQDs/BiOBr.
|
Download:
|
| Fig. 1. The high magnification SEM image (a), TEM image (b), high magnification TEM image and EDS (d) of the 3.1wt% CQDs/BiOBr. | |
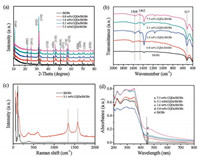
XRD patterns of the pristine BiOBr and as-prepared CQDs/BiOBr composites are shown in Fig. 2a. All CQDs/BiOBr samples with different CQDs concentrations present strong and well-resolved diffraction peaks same as the pristine BiOBr, which are in a good agreement with tetragonal BiOBr (JCPDS 73-2061). The characteristic peak for carbon at 26° is too weak to be observed, which may attribute to its low concentration in the composites [28]. No other characteristic peaks were found, indicating no impurity existing in the as-prepared samples. Fig. 2b shows the FT-IR spectra of the pristine BiOBr and the CQDs/BiOBr composite photocatalysts. The absorption peak at around 1568 cm-1 is assigned to the C=O stretching vibration. The band at 1462cm-1 is attributed to the -COO- [33]. It indicates that -COOH groups dominate the surface of CQDs. The peak at 517 cm-1 is associated with the Bi-O stretching mode. Combining XRD and FT-IR analyses, we can confidentlyconclude that BiOBr and CQD are integrated together to form the composite catalysts. Raman spectra of the pristine BiOBr and 3.1 wt% CQDs/BiOBr composite are shown in Fig. 2c. The two characteristic peaks located at about 1340 and 1580 cm-1 are observed in the 3.1 wt% CQDs/BiOBr composite, which correspond to the D-band and G-band of carbon, respectively, similar to the previous reports [26, 28].
|
Download:
|
| Fig. 2. XRD patterns (a) and FT-IR analysis (b) of BiOBr and CQDs/BiOBr materials with different contents of CQDs; (c) Raman analysis of 3.1 wt% CQDs/BiOBr and pure BiOBr materials; (d) UV–vis diffuse reflectance spectra of the as-prepared samples. | |
The UV–vis absorption spectra of pristine BiOBr and CQDs/BiOBr composites are collected to understand the band structure of these photocatalysts. It can be seen that the BiOBr sample shows a fundamental absorption edge rising at 430 nm, in line with the previously reported result [34]. As demonstrated in Fig. 2d, with CQDs content increases from 0.8wt% to 7.5wt% the absorption intensity in the range of 430–800 nm of the as-prepared samples increases gradually. As aforementioned, the CQDs/BiOBr composites exhibit a higher photocatalytic activity than the pristine BiOBr for target reactions under visible light, indicating CQDs play important roles in utilizing sunlight. As seen from Fig. S1 in Supporting information, the band gap of pure BiOBr was about 2.77 eV. For the composite system, the variation of band gaps of BiOBr in CQDs/BiOBr composites can be ignored. The addition of CQDs may lead to a better response in the visible light region and therefore a more efficient electron-hole pair separation.
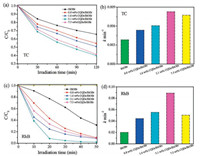
To evaluate the photocatalytic activities of the CQDs/BiOBr composite photocatalysts with various CQDs contents, photocatalytic experiments are carried out by degradation of colorless antibiotic TC, which is difficult to be decomposed naturally. As shown in Fig. 3a, after 120 min irradiation, the pristine BiOBr can only remove about 30% of TC, while all the CQDs/BiOBr composites show an enhanced photocatalytic performance. It is observed 3.1 wt% CQDs/BiOBr composite has the highest photocatalytic activity for TC removal under visible light irradiation, with only 40% of TC left after 120 min irradiation. The photocatalytic performance decreased after introducing excessive CQDs in BiOBr, it is because too many electron sinks can reversely increase the hole and electron recombination [35]. We further investigated the photodegradation of RhB under visible light irradiation. It is clearly seen in Fig. 3c that direct RhB photolysis without any catalyst is negligible, while the CQDs/BiOBr composite photocatalysts shows a higher photocatalytic activity compared with the pristine BiOBr, with the 3.1 wt% CQDs/BiOBr being the most active. After 20 min's irradiation, 91.8% of RhB is degraded by 3.1 wt% CQDs/BiOBr, in contrast, only 22.7% of RhB is catalytically removed if using unmodified BiOBr in the same condition. After irradiation for 50 min, all CQDs/BiOBr composite photocatalysts is capable of degradeing more than 90% of RhB. To further evaluate the benefits of introducing CQDs stock solution instead of CQDs powders, photocatalytic degradation of BPA have been conducted and the degradation curves are shown in Fig. S2 in Supporting information. After reaction for 150 min, 25.4%, 29.7%, 73% and 50.1% BPA are degraded by 0.8 wt% CQDs/BiOBr, 1.6 wt% CQDs/BiOBr, 3.1 wt% CQDs/BiOBr and 7.5 wt% CQDs/BiOBr, respectively. The detailed photodegradation process is presented in Figs. S2b-e. In our previous work of freeze-dried powder synthesized CQDs/BiOBr, only 48% of BPA is removed on 3 wt% CQDs/BiOBr under the same catalytic condition, which is interior to that (73%, on 3.1 wt% CQDs/BiOBr) in the present work. These experimental results indicated the strong interaction between CQDs and BiOBr and the better CQDs dispertion in the composites will enhance the photocatalytic performance.
|
Download:
|
| Fig. 3. Photodegradation of TC (a) and RhB (c) over CQDs/BiOBr with different CQDs content and pure BiOBr under visible light irradiation; Degradation rate of TC (b) and RhB (d) with the pure BiOBr and CQDs/BiOBr samples. | |
Figs. 3b and d show the degradation rate of TC and RhB degradation by pristine BiOBr and CQDs/BiOBr composite catalysts. A pseudo-first-order reaction model is applied to describe the experimental data as follows: -ln(C0/C) = kapt, where kap is the pseudo-first-order rate constant, C0 is the adsorption equilibrium concentration of TC or RhB, t is the reaction time, and C is the concentration of TC or RhB at the reaction time t (Fig. S3 in Supporting information). The pseudo-first-order rate constants and relative coefficients are summarized in Table S1 and Table S2 in Supporting information. It is noted that the rate constant kap exhibits a volcano-like trend along with the increase of CQDs concentration. The maximum rate constant kap was observed on 3.1 wt% CQDs/BiOBr, which is 0.0083 min-1 for TC and 0.089 min-1 for RhB, 2 times and 4 times higher than that of pristine BiOBr.
We further performed photoelectrochemical experiments to investigate the electronic interaction between CQDs and BiOBr. It is widely accepted that a higher photocurrent can be correlated to a better separation efficiency of the electrons and holes within photocatalyst, and hence suggests a higher photocatalytic activity [36]. The transient photocurrent responses of the pristine BiOBr and as-prepared 3.1 wt% CQDs/BiOBr electrodes are recorded for several on-off cycles of visible light irradiation and are shown in Fig. 4a. Fast and stable photocurrent responses were detected for each switch-on and -off event on both electrodes. When the illumination is stopped, the current returned rapidly to its dark current state. It can be seen that the photocurrent of the 3.1 wt% CQDs/BiOBr electrode is about 2.5 times higher than that of the BiOBr, which confirms the enhanced separation efficiency of photoinduced electrons and holes. Such an enhancement can be attributed to the function of CQDs, which works as electron collectors to trap electrons generated in BiOBr nanosheets.

|
Download:
|
| Fig. 4. Transient photocurrent response (a), EIS profiles (b), PL spectra (c) of pure BiOBr and 3.1 wt% CQDs/BiOBr samples; (d) Photocatalytic degradation of RhB over 3.1 wt% CQDs/BiOBr without and with the additon of hole and radical scavenger. | |
EIS measurement was also employed to investigate the separation process of photogenerated electrons and holes. Fig. 4b presents Nyquist plots of ITO/BiOBr and ITO/CQDs/BiOBr, which shows a smaller arc radius on the ITO/CQDs/BiOBr, indicating that 3.1 wt% CQDs/BiOBr has the lower resistance than pristine BiOBr. Hence, it is concluded that an enhanced separation of the photogenerated electrons and holes and a fast interfacial charge transfer are obtained by compositing BiOBr with CQDs. CQDs could work as transporters for photogenerated electrons, prolonging the lifetime of the charge carriers. This can also be confirmed by PL spectra of the pristine BiOBr and 3.1 wt% CQDs/BiOBr as shown in Fig. 4c. Excited at 360 nm, a decrease in the PL intensity is observed on 3.1 wt% CQDs/BiOBr, compared to pristine BiOBr, suggesting that the 3.1 wt% CQDs/BiOBr has a lower recombination probability of photogenerated electrons and holes than pristine BiOBr [36].
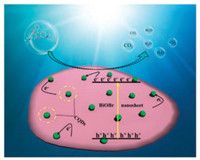
Fig. 4d shows the photocatalytic degradation of RhB with the addition of hole and hydroxyl radical scavengers, respectively. With the addition of 1 mmol tert-butanol as the hydroxyl radical scavenger, only a slight decrease in the photocatalytic activity is observed, while the RhB photodegradation is greatly inhibited with the addition of hole scavenger (EDTA-2Na) [37]. This result suggests that the direct hole oxidation reaction is the dominant photocatalytic process. The significant enhancement of photodegradation of TC and RhB is attributed to the synergistic effect between CQDs and BiOBr nanosheets (Fig. 5). The CQDs function as an electron collector and transporter to promote charge transfer and separation, suppressing the recombination probability and improving the lifetime of electron-hole pairs [28].
|
Download:
|
| Fig. 5. Schematic of the separation and transfer of photogenerated charges in the CQDs/BiOBr combined with the possible reaction mechanism of photocatalytic procedure. | |
In conclusion, novel CQDs/BiOBr composite photocatalysts were prepared via a facile hydrothermal method in the presence of reactive ionic liquid [C16mim]Br. By directly employing the CQDs stock solution in the synthesis without the freeze-drying step that reported in our previous work [32], the structural stability and photocatalytic activity of CQDs/BiOBr composites was significantly enhanced under visible light irradiation. Among all the CQDs/BiOBr, the 3.1 wt% CQDs/BiOBr demonstrated the highest photocatalytic activity for the degradation of TC and RhB under visible light irradiation, which was about 2 times and 4 times higher than that of pristine BiOBr, respectively. Based on photoelectrochemical results and PL analysis, a photocatalytic mechanism was proposed and well-discussed. The enhanced photocatalytic performance can be attributed to the high separation efficiency of the photogenerated electron-holes pairs under the assistance of CQDs. Our results have provided a new strategy to design a new class of CQDsbased photocatalysts for energy and environmental applications.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21471069, 21476098 and 21576123), Jiangsu University Scientific Research Funding (No. 11JDG0146), and Doctoral Innovation Fund of Jiangsu Province (No. KYCX17_1791).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.05.002.
| [1] |
Z.G. Zou, J.H. Ye, K. Sayama, et al., Nature 414 (2001) 625-627. DOI:10.1038/414625a |
| [2] |
M.Z. Ge, Q.S. Li, C.Y. Cao, et al., Adv. Sci. 4 (2017) 1600152. DOI:10.1002/advs.201600152 |
| [3] |
J. Li, X.Y. Wu, W.F. Pan, et al., Angew. Chem. Int. Ed. 57 (2018) 491-495. DOI:10.1002/anie.201708709 |
| [4] |
X.G. Han, Q. Kuang, M.S. Jin, et al., J. Am. Chem. Soc. 131 (2009) 3152-3153. DOI:10.1021/ja8092373 |
| [5] |
A. Mclaren, T. Valdes-Solis, G.Q. Li, et al., J. Am. Chem. Soc. 131 (2009) 12540-12541. DOI:10.1021/ja9052703 |
| [6] |
H.N. He, D. Sun, Q. Zhang, et al., ACS Appl. Mater. Interfaces 9 (2017) 6093-6103. DOI:10.1021/acsami.6b15516 |
| [7] |
X. Chen, L. Liu, P.Y. Yu, et al., Science 331 (2011) 746-750. DOI:10.1126/science.1200448 |
| [8] |
G.H. Qin, Z. Sun, Q.P. Wu, et al., J. Hazard. Mater. 192 (2011) 599-604. DOI:10.1016/j.jhazmat.2011.05.059 |
| [9] |
C. Han, Z. Chen, N. Zhang, et al., Adv. Funct. Mater. 25 (2015) 221-229. DOI:10.1002/adfm.v25.2 |
| [10] |
P. Li, Z. Wei, T. Wu, et al., J. Am. Chem. Soc. 133 (2011) 5660-5663. DOI:10.1021/ja111102u |
| [11] |
Y.L. Liu, J. Di, M.X. Ji, et al., J. Colloid Interf. Sci. 492 (2017) 25-32. DOI:10.1016/j.jcis.2016.12.026 |
| [12] |
J. Li, Y. Yu, L.Z. Zhang, Nanoscale 6 (2014) 8473-8488. DOI:10.1039/C4NR02553A |
| [13] |
H.F. Cheng, B.B. Huang, Y. Dai, Nanoscale 6 (2014) 2009-2026. DOI:10.1039/c3nr05529a |
| [14] |
J.X. Xia, J. Di, S. Yin, et al., RSC Adv. 4 (2014) 82-90. DOI:10.1039/C3RA44191A |
| [15] |
H. Li, J. Shang, Z.H. Ai, et al., J. Am. Chem. Soc. 137 (2015) 6393-6399. DOI:10.1021/jacs.5b03105 |
| [16] |
J. Di, J.X. Xia, M.X. Ji, et al., ACS Sustain. Chem. Eng. 4 (2016) 136-146. DOI:10.1021/acssuschemeng.5b00862 |
| [17] |
J. Di, J.X. Xia, M.X. Ji, et al., J. Mater. Chem. A 3 (2015) 15108-15118. DOI:10.1039/C5TA02388B |
| [18] |
S.Y. Wang, X. Hai, X. Ding, et al., Adv. Mater. (2017), 1701774. |
| [19] |
C.Y. Wang, X. Zhang, H.B. Qiu, et al., Appl. Catal. B 205 (2017) 615-623. DOI:10.1016/j.apcatb.2017.01.015 |
| [20] |
J.X. Xia, J.D.S. Yin, et al., Mater. Sci. Semicon. Proc. 24 (2014) 96-103. DOI:10.1016/j.mssp.2014.02.036 |
| [21] |
X.M. Jia, J. Cao, H.L. Lin, et al., Appl. Catal. B 204 (2017) 505-514. DOI:10.1016/j.apcatb.2016.11.061 |
| [22] |
R.J. Hou, Y. Gao, H.J. Zhu, et al., Chem. Eng. J. 317 (2017) 386-393. DOI:10.1016/j.cej.2017.02.085 |
| [23] |
G.H. Jiang, X.H. Wang, Z. Wei, et al., J. Mater. Chem. A 1 (2013) 2406-2410. DOI:10.1039/c2ta00942k |
| [24] |
S.J. Zhu, Q.N. Meng, L. Wang, et al., Angew. Chem. 52 (2013) 3953-3957. DOI:10.1002/anie.v52.14 |
| [25] |
H.T. Li, Z.H. Kang, Y. Liu, et al., J. Mater. Chem. 22 (2012) 24230-24253. DOI:10.1039/c2jm34690g |
| [26] |
J. Tian, Y.H. Leng, Z.H. Zhao, et al., Nano Energy 11 (2015) 419-427. DOI:10.1016/j.nanoen.2014.10.025 |
| [27] |
L.Z. Li, C.H. Liu, Y.Y. Qiu, et al., Int. J. Hydrogen Energy 42 (2017) 19654-19663. DOI:10.1016/j.ijhydene.2017.06.078 |
| [28] |
H.C. Zhang, H. Huang, H. Ming, et al., J. Mater. Chem. 22 (2012) 10501-10506. DOI:10.1039/c2jm30703k |
| [29] |
H.T. Li, R.H. Liu, Y. Liu, et al., J. Mater. Chem. 22 (2012) 17470-17475. DOI:10.1039/c2jm32827e |
| [30] |
K.H. Ye, Z.L. Wang, J.W. Gu, et al., Energy Environ. Sci. 10 (2017) 772-779. DOI:10.1039/C6EE03442J |
| [31] |
F.L. Wang, Y.F. Wang, Y.P. Feng, et al., Appl. Catal. B 221 (2018) 510-520. DOI:10.1016/j.apcatb.2017.09.055 |
| [32] |
J.X. Xia, J. Di, H.T. Li, et al., Appl. Catal. B 181 (2016) 260-269. DOI:10.1016/j.apcatb.2015.07.035 |
| [33] |
J.Z. Li, N.Y. Wang, T.T. Tran, et al., Analyst 138 (2013) 2038-2043. DOI:10.1039/c3an36653g |
| [34] |
Z. Jiang, F. Yang, G. Yang, et al., J. Photochem. Photobiol. A 212 (2010) 8-13. DOI:10.1016/j.jphotochem.2010.03.004 |
| [35] |
J. Di, J.X. Xia, M.X. Ji, et al., Langmuir 32 (2016) 2075-2084. DOI:10.1021/acs.langmuir.5b04308 |
| [36] |
M.X. Ji, J.X. Xia, J. Di, et al., Chem. Eng. J. 331 (2018) 355-363. DOI:10.1016/j.cej.2017.08.100 |
| [37] |
M.X. Ji, J. Di, Y.P. Ge, et al., Appl. Surf. Sci. 413 (2017) 372-380. DOI:10.1016/j.apsusc.2017.03.287 |
 2018, Vol. 29
2018, Vol. 29 


