Photoelectrochemical (PEC) water splitting has been considered as a promising technology for storing solar energy [1]. Since the start-up work of Fujishima and Honda on a TiO2 photoanodes, many visible-light responsive semiconductor materials, such as α-Fe2O3, Ta3N5, and BiVO4, have attracted intensive attention from researchers [2-4]. Among these materials, α-Fe2O3 is one of the most promising photoanode materials due to its high theoretical efficiency (about 16%), excellent photochemical stability, nontoxicity and low cost [5]. However, the PEC performance of α-Fe2O3 is hindered by some intrinsic drawbacks, including short hole diffusion length, poor electrical conductivity, severe surface states recombination and so on.
In order to address these limitations and achieve higher PEC performance, many methods have been carried out over decades [6-14]. Element doping with high valent cations such as Ti, Si, or Sn markedly enhances the plateau photocurrent of hematite photoanodes, due to the improvement of electron concentration and n-type conductivity [9, 15, 16]. High temperature annealing is another common strategy for targeting a larger photocurrent of hematite photoanodes by increasing crystallinity and activating dopants [17]. A combination of doping and high temperature annealing strategies can further improve the plateau photocurrent of hematite photoanodes, compared with applying a single strategy [16, 18]. However, an unfavorable onset potential shift of doped hematite photoanodes appeared after high temperature annealing. For this phenomenon of Ti doped hematite photoanodes, to the best of our knowledge, the underlying mechanism has not been explored to date and it deserves further investigation.
In this study, we have used different photoelectrochemical and structural characterization techniques to confirm that the change of onset potential comes from a surface effect. Through a combination of X-ray photoelectron spectroscopy (XPS) measurements and density functional theory plus Hubbard U correction (DFT + U) calculations, we further revealed that the surface Ti/Fe atomic ratio increased after high temperature annealing, which would decrease the amount of surface adsorbed hydroxide ions. As a result, the flatband potential (i.e., the theoretical onset potential) of Ti doped hematite photoanodes would positively shift after high temperature annealing, proven by Mott-Schottky measurements.
Herein, the titanium doped hematite photoanodes (denoted as Ti:Fe2O3) were prepared using a previously established method [19]. High temperature (denoted as HT in all figures of this study) annealing was performed on the as-prepared hematite film at 750 ℃ for 10 min. Fig. 1 presents the photocurrent-potential curves of Ti:Fe2O3 before and after high temperature annealing. After high temperature annealing, the plateau photocurrent of Ti:Fe2O3 doubled, indicating the enhanced photoactivity [17, 20]. Meanwhile, the photocurrent onset potential was significantly shifted anodically from 0.95 VRHE to 1.05 VRHE. This unfavorable shift suppressed the PEC response of Ti:Fe2O3 under low bias, compared to that of the samples without high temperature annealing treatment. Similar phenomena have been also observed in previous literatures [18, 20], which is consistent with this study.

|
Download:
|
| Fig. 1. Photocurrent density vs. potential curves of as-prepared (blue) and HTannealed (red) Ti:Fe2O3 photoanodes, under H2O oxidation condition. Dark current vs. potential curves are of dash lines. | |
X-Ray diffraction (XRD) data of Ti:Fe2O3 before and after high temperature annealing are shown in Figs. S1a and b (Supporting information), confirming the presence of hematite (JCPDS No. 330664). Peak positions and intensities are similar for both samples, indicating annealing treatment does not change the phase and crystallinity. Also, the grain size of both samples are almost the same, as determined by the full width at half-maximum (FWHM) of the (110) diffraction peaks. Figs. 2a and b show the scanning electron microscopy (SEM) surface images of Ti:Fe2O3 before and after high temperature annealing, respectively. No obvious changes of surface morphology and grain size were observed. Some nanoparticles are fused together and both of the samples exhibits crack structures, matching with the literature [19]. Additionally, the UV–vis absorption spectra were measured and the results are in Fig. 2c, suggesting no difference in the light absorption capability upon the high temperature annealing. Taken together, it is impossible that the unfavorable onset shift comes from the unchanged phase, crystallinity, grain size, morphology and light absorption capability. Moreover, control experiments showed that annealing at 750 ℃ for 10 min would not have a measurable change on the conductivity of fluorine-doped tin oxide (FTO) substrate, in line with previous reports [21].

|
Download:
|
| Fig. 2. (a), (b) SEM images of as-prepared and HT-annealed Ti:Fe2O3 film samples, respectively. (c) UV–vis absorption spectra for as-prepared (blue) and HT-annealed Ti:Fe2O3 (red). (d) Chopped photocurrent density vs. potential curves of as-prepared (blue) and HT-annealed (red) Ti:Fe2O3, under H2O2 oxidation condition. | |
Further, in order to separate any impact due to bulk change from the surface effects, photocurrent-potential measurements were carried out in the presence of an efficient hole scavenger (H2O2). The onset potential of both photoanodes in contact with H2O2 are identical shown in Fig. 2d, which indicates the difference of water oxidation onset potential of photoanodes before and after high temperature annealing is a consequence of surface effect [22, 23].
XPS measurements were used to characterize the composition change on the surface of the films before and after high temperature annealing, which would provide additional information about surface changes [24, 25]. Fig. 3 shows the binding energy of O 1s, Fe 2p, Ti 2p and Sn 3d for both samples. The peaks at 529.7 eV and 710.5 eV are assigned to the O2- and Fe3+ ions in hematite, respectively [20]. Both photoanodes show the binding energy of Ti 2p at the peak of 457.9 eV, implying that Ti cations are in a different atomic environment from that in pure TiO2 (binding energy of 459.0 eV) [20]. No signal of Sn4+ was detected on the surface of both samples, evincing that there is no Sn4+ on the hematite surface due to the diffusion from the FTO substrate. It should be noted that the Ti/Fe atomic ratio on the surface has an apparent increase after high temperature annealing obtained from the XPS data. Another notable change after high temperature annealing is the amount decrease of hydroxyl group (—OH) on the surface, as determined by the decrease of the peak intensities at about 532.5 eV [2, 26]. This was in accordance with Cao et al.'s work [27], when titanium was intentionally introduced into hematite lattices.

|
Download:
|
| Fig. 3. XPS spectra of as-prepared (blue) and HT-annealed (red) Ti:Fe2O3. (a) Fe 2p, (b) Ti 2p, (c) O 1s, (d) Sn 3d. | |
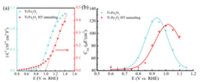
MoBT-Schottky analysis was performed to gain further insight into changes after the high temperature annealing. Fig. 4a exhibits Mott-Schottky plots of both samples and the Vfb (denoted as flatband potential) of Ti:Fe2O3 positively shifted about 100 mV after high temperature annealing. Vfb was denoted as the theoretical onset potential, as one would expect a photocurrent at any potentials positive of Vfb theoretically [28]. So it reveals that it is the positive shift of Vfb that induce the unfavorable onset potential after high temperature annealing.
|
Download:
|
| Fig. 4. (a) Mott-Schottky plots of as-prepared (blue) and HT-annealed (red) Ti: Fe2O3 samples. (b) Css values of as-prepared (blue) and HT-annealed (red) Ti:Fe2O3 samples obtained from PEIS measurement. | |
According to previous reports, a shift of flatband potential on hematite photoanodes is generally attributed to changes of surface states and/or surface ions adsorption [19, 29]. Firstly, in order to investigate the effects of high temperature annealing on the surface states properties, we carried out photoelectrochemical impedance spectra (PEIS) measurements of the photoanodes before and after high temperature annealing. Representative Nyquist plots shown in Fig. S2 (Supporting information) exhibits apparently two semicircles and the low frequency semicircle is attributed to the surface states. The values for Css (surface states capacitance) extracted from fitting the PEIS data under different biases are displayed in Fig. 4b, showing Gaussian behavior for both photoanodes before and after high temperature annealing [30]. While the peak intensity of Css did not have an apparent decrease/ increase after high temperature annealing, the peak shifted 100 mV to a more positive potential, in good agreement with the shift of Vfb. Therefore, it suggests that the amount and relative location of surface states in band gap does not change after annealing treatment and surface states of Ti:Fe2O3 are not responsible for the change of Vfb and onset potential.
Secondly, we will investigate the effect of surface ions' adsorption on the flatband potential shift. When a metal oxide photoelectrode is immersed in an aqueous solution, H+ and OH- ions in the solution will successively adsorb/desorb from the surface and form a region called Helmholtz layer. The amount of adsorbed H+ and OH- ions on the electrode surface (i.e., inner Helmholtz layer) would change the potential drop across the Helmholtz layer and then shift the flatband potential of the electrode [31]. With a testing electrolyte pH of 13.6, the net total charge adsorbed at a hematite photoanode should be negative. Reducing the amount of surface adsorbed OH- ions would then decrease the Helmholtz voltage and positively shift the flatband potential of hematite photoanodes.
Recalling the XPS data, the amount decrease of surface hydroxyl group (i.e., adsorbed OH- ions) was observed after high temperature annealing, which could induce the anodic shift of flatband potential and onset potential. At the same time, we note that the decrease of surface hydroxyl group is correlated to the increase of surface Ti doping. Similar phenomenon was obtained in previous work [32]. To furtherly investigate whether it would decrease the hydroxide ions' adsorption capacity when inducing more Ti doping on the surface, we applied DFT computations to calculate the adsorption energy change before and after introducing Ti atom on the hematite surface.
Two surface models, (110) and (110) + TiFe, were adopted to perform the OH- adsorption energy calculations. Since PEC water oxidation of hematite electrodes is under high pH condition, here we used oxygen-terminated (110) surfaces, at which the surfaces were deprotonated [33]. For Ti doped hematite surface model, we denoted the surface substitutional Ti atom as a center and calculated OH- adsorption energies bonding with the central Ti atom and nearest-neighbor Fe atoms on the surface, including 3 terminal sites and 3 bridge sites. Similar calculation were performed on the pristine (110) surface model. Figs. 5a and b show the most stable structures of OH- bonding with the central Ti/Fe atom on (110) and (110) + TiFe surfaces, respectively. Table 1 lists adsorption energies of one OH- bonding with different reaction sites on these two surface models. The OH- adsorption energies on (110) surface are always smaller than that on the (110) + TiFe surface, suggesting that Ti doping would decrease the OH- adsorption capacity on the hematite (110) surface, in line with our XPS data. Our study reveals that, due to the surface Ti/Fe atomic ratio increase caused by high temperature annealing, the surface OH- adsorption capacity would decrease and it would induce the anodic shift of flatband potential and the unfavorable onset shift. We hope that the future investigations on Ti doped hematite photocatalyst may be enlightened from this work and the unfavorable onset shift caused by high temperature annealing can be overcome.

|
Download:
|
| Fig. 5. The relaxed atomic structures of hydroxide ion bonding with the central Ti/ Fe atom on the (a) (110) and (b) (110) + TiFe surfaces (upper: side-view; lower: topview). The yellow, blue, red, white balls are Fe, Ti, O, H atoms, respectively. | |
|
|
Table 1 Adsorption energies (Eads in eV) for OH- adsorption bounding with different metal sites on the (110) and (110) + TiFe surfaces. |
In conclusion, we have investigated the effect of high temperature annealing on the water splitting onset potential of Ti:Fe2O3 photoanodes. XPS measurements have shown that surface Ti/Fe atomic ratio increases and adsorbed hydroxide ions amount decreases, as a result of high temperature annealing. Further DFT calculation suggests that more surface Ti doping would decrease the surface OH- adsorption energy, which is in line with the XPS data. The following flatband potential shift caused by hydroxide ions adsorption capacity change was also observed under Mott-Ti:Fe2O3 photoanodes.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (Nos. 21473090, U1663228). Supercomputing facilities of the High Performance Computing Center (HPCC) of Nanjing University are also acknowledged.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.01.022.
| [1] |
K. Sivula, F. Le Formal, M. Gratzel, ChemSusChem 4 (2011) 432-449. DOI:10.1002/cssc.201000416 |
| [2] |
Y. He, J.E. Thorne, C.H. Wu, et al., Chem 1 (2016) 640-655. DOI:10.1016/j.chempr.2016.09.006 |
| [3] |
Y. Hu, Y. Wu, J. Feng, et al., J. Mater. Chem. A 6 (2018) 2568-2576. DOI:10.1039/C7TA10361A |
| [4] |
G. Wang, Y. Ling, D.A. Wheeler, et al., Nano Lett. 11 (2011) 3503-3509. DOI:10.1021/nl202316j |
| [5] |
A. Kay, I. Cesar, M. Gratzel, J. Am. Chem. Soc. 128 (2006) 15714-15721. DOI:10.1021/ja064380l |
| [6] |
K. Sivula, F.L. Formal, M. Gratzel, Chem. Mater. 21 (2009) 2862-2867. DOI:10.1021/cm900565a |
| [7] |
D.K. Zhong, J. Sun, H. Inumaru, D.R. Gamelin, J. Am. Chem. Soc. 131 (2009) 6086-6087. DOI:10.1021/ja9016478 |
| [8] |
S.D. Tilley, M. Cornuz, K. Sivula, M. Gratzel, Angew. Chem. Int. Ed. 49 (2010) 6405-6408. DOI:10.1002/anie.201003110 |
| [9] |
M. Zhang, W. Luo, Z. Li, et al., Appl. Phys. Lett. 97 (2010) 042105. DOI:10.1063/1.3470109 |
| [10] |
T. Hisatomi, J. Brillet, M. Cornuz, et al., Faraday Discuss. 155 (2012) 223-232. DOI:10.1039/C1FD00103E |
| [11] |
T.Y. Yang, H.Y. Kang, U. Sim, et al., Phys. Chem. Chem. Phys. 15 (2013) 2117-2124. DOI:10.1039/c2cp44352j |
| [12] |
Z. Fu, T. Jiang, L. Zhang, et al., J. Mater. Chem. A 2 (2014) 13705-13710. DOI:10.1039/C4TA02527J |
| [13] |
Y. Qiu, S.F. Leung, Q. Zhang, et al., Nano Lett. 14 (2014) 2123-2129. DOI:10.1021/nl500359e |
| [14] |
I.S. Cho, H.S. Han, M. Logar, et al., Adv. Energy Mater. 6 (2016) 1501840. DOI:10.1002/aenm.201501840 |
| [15] |
N.T. Hahn, C.B. Mullins, Chem. Mater. 22 (2010) 6474-6482. DOI:10.1021/cm1026078 |
| [16] |
Y. Ling, G. Wang, D.A. Wheeler, et al., Nano Lett. 11 (2011) 2119-2125. DOI:10.1021/nl200708y |
| [17] |
J. Brillet, M. Gratzel, K. Sivula, Nano Lett. 10 (2010) 4155-4160. DOI:10.1021/nl102708c |
| [18] |
A.J. Abel, I. Garcia-Torregrosa, A.M. Patel, et al., J. Phys. Chem. C 119 (2015) 4454-4465. DOI:10.1021/jp510027u |
| [19] |
D. Cao, W. Luo, J. Feng, et al., Energy Environ. Sci. 7 (2014) 752-759. DOI:10.1039/C3EE42722F |
| [20] |
J. Deng, J. Zhong, A. Pu, et al., J. Appl. Phys. 112 (2012) 084312. DOI:10.1063/1.4759278 |
| [21] |
J.Y. Kim, G. Magesh, D.H. Youn, et al., Sci. Rep. 3 (2013) 2681. DOI:10.1038/srep02681 |
| [22] |
H. Dotan, K. Sivula, M. Grätzel, et al., Energy Environ. Sci. 4 (2011) 958-964. DOI:10.1039/C0EE00570C |
| [23] |
O. Zandi, T.W. Hamann, J. Phys. Chem. Lett. 5 (2014) 1522-1526. DOI:10.1021/jz500535a |
| [24] |
J. Fang, F. Shi, H. Bao, et al., Chin. J. Catal. 34 (2013) 2075-2083. DOI:10.1016/S1872-2067(12)60667-6 |
| [25] |
H. Bao, K. Qian, J. Fang, W. Huang, Appl. Surf. Sci. 414 (2017) 131-139. DOI:10.1016/j.apsusc.2017.04.018 |
| [26] |
J. Ding, Q. Zhong, S. Zhang, RSC Adv. 4 (2014) 5394-5398. DOI:10.1039/c3ra46418k |
| [27] |
D. Cao, W. Luo, M. Li, et al., CrystEngComm 15 (2013) 2386-2391. DOI:10.1039/c3ce26811j |
| [28] |
R. van de Krol, M. Grätzel, Photoelectrochemical Hydrogen Production, Springer, New York, 2012.
|
| [29] |
L. Steier, I. Herraiz-Cardona, S. Gimenez, et al., Adv. Funct. Mater. 24 (2014) 7681-7688. DOI:10.1002/adfm.v24.48 |
| [30] |
B. Klahr, S. Gimenez, F. Fabregat-Santiago, et al., J. Am. Chem. Soc. 134 (2012) 4294-4302. DOI:10.1021/ja210755h |
| [31] |
J. Bisquert, P. Cendula, L. Bertoluzzi, S. Gimenez, J. Phys. Chem. Lett. 5 (2014) 205-207. DOI:10.1021/jz402703d |
| [32] |
J. Fang, X. Bi, D. Si, et al., Appl. Surf. Sci. 253 (2007) 8952-8961. DOI:10.1016/j.apsusc.2007.05.013 |
| [33] |
X. Zhang, P. Klaver, R. van Santen, et al., J. Phys. Chem. C 120 (2016) 18201. DOI:10.1021/acs.jpcc.6b07228 |
 2018, Vol. 29
2018, Vol. 29