b Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
Along with the development of nanotechnology, nano-catalysts play an increasingly important role in the field of catalysis due to their high efficiency and unique properties compared with the corresponding bulk catalysts [1-8]. However, a serious obstacle for practical utilization of nano-catalysts is the recycling and separation of the catalysts after chemical reaction, especially in multi-phasic systems [9-11]. Though traditional filtration or centrifugation methods can be used, these operations will inevitably lead to the loss of catalyst during transferring the reaction solution from one vessel to another. Moreover, these methods often involve the consumption of extra energy in particularly when the particle size is very small [12, 13]. Magnetic field assisted isolation is one of the relatively effective ways to combat these problems, but external magnetic fields are required [14-18]. Therefore, exploring more efficient methods for achieving the easy recovery and separation of nanoparticle catalysts are still highly desirable.
Recently, our group developed a novel pH-switched Pickering emulsion catalytic system for multi-phase reactions [19]. In this system, an interfacially active silica nanoparticle prepared with a pH-sensitive (MeO)3-SiCH2CH2CH2(NHCH2CH2)2NH2 group on the surface was utilized. The surface hydrophilic/hydrophobic properties of the silica emulsifier can be easily altered by tuning pH values, leading to the reversible circulation process of emulsification/demulsification and achieving the recovery and reuse of the solid catalyst. Because of the large interface area in the Pickering emulsions, the interfacial reaction efficiency could be improved by several orders of magnitude compared to bulk systems. Such a new strategy might provide new opportunities for separating and recycling solid catalysts, however, still less studied as far as we know.
On the other hand, for pH-switched Pickering emulsion systems, silica or organic polymers are often used as the solid stabilizers [20-23], more diverse of useful solid emulsifier are expected to be developed. Titanium dioxide (TiO2) is a traditional support in catalysis and also a potential photocatalyst due to its low cost, safety, resistance to acid and base and highly photocatalysis activity [24-28]. It has been widely used in photocatalytic degradation of organics in water and water splitting to generate hydrogen [29-33]. However, there are little reports for its application in Pickering emulsion catalytic system [34-38], especially in pH-responsive system.
Herein, in this work, we further investigated the pH responsive Pickering emulsion system for in situ catalyst recycling using interficially active TiO2 catalyst nanoparticles as emulsifier. As illustrated in Scheme 1a, the surface wettability of the catalyst can be switched by adding acid or base. At the beginning of reaction, an oil-in-water (O/W) Pickering emulsion system was formed using amino-functionalized TiO2 catalysts as stabilizer (Scheme 1b). Because of the increased interface area, the catalytic efficiency could be improved. After reaction, the emulsions were broken completely by adding a small amount acid in the system. The organic phase in the upper layer can be isolated by simple phase separation, while the solid catalysts residing in water phase will be directly used for the next reaction cycle after tuning the pH by the addition of base.

|
Download:
|
| Scheme 1. Schematic illustration of the pH-switched Pickering emulsion strategy. | |
The interfacially active TiO2 nanoparticles were prepared via a simple post-grafting method. In a typical procedure, 1.0 g of TiO2 nanoparticles (dried at 100 ℃ for 1 h) were dispersed into 5 mL of toluene. Then, a given amount of 3-(aminopropyl)tri-methoxysilane (APTMS) and triethylamine (The mole ratio of APTMS and triethylamine were 1:1) were added into this suspension. After stirring at 110 ℃ for 6 h, the resultant solid material was isolated by centrifugation. After being washed four times with toluene and dried at 40 ℃ under vacuum. The aminopropyl-modified TiO2 nanoparticles were obtained. In order to investigate the influence of surface property of the functionalized particles, the amount of APTMS was varied from 0.5 mmol, 1.0 mmol, 1.5 mmol and 2.0 mmol, the resultant particles were denoted as TiO2-N (x) (x = 0.5, 1.0, 1.5 and 2.0, respectively).
The morphology and composition of the interfacially active TiO2 nanoparticles were characterized with transmission electron microscopy (TEM), Fourier transform infrared (FT-IR), X-ray photoelectron spectra (XPS), and thermal gravimetric analysis (TGA) using TiO2-N (1.0) as a representative sample.
The TEM image of TiO2-N shows that the sample consists of rodlike nanoparticles with diameters around 10–15 nm and length around 20–45 nm (Fig. 1a). In order to confirm the successful introduction of amino-functional groups, FT-IR was employed to determine the surface composition of the modified particles. As shown in Fig. 1b, comparing with pure TiO2, there are some new peaks appear in the FT-IR spectra of TiO2-N. The peaks located at 2927 and 2854 cm-1 can be attributed to the C—H stretching vibration of the grafted aminopropyl group. In addition, the peaks at 1000–1200 cm-1 are originated from the stretching vibration of the Si—O—Si bond. These observations demonstrate that the aminopropyl group is successfully grafted on the surface of TiO2 nanoparticles. Fig. 1c shows the XPS elemental survey scans of TiO2-N, the peaks corresponding to C, N, O, Si and Ti elements are clearly observed, which further confirmed the successful linking of organic functional groups on the TiO2 surface.

|
Download:
|
| Fig. 1. (a) TEM image of TiO2-N; (b) FTIR spectra of TiO2 and TiO2-N; (c) XPS spectra of TiO2-N; (d) TG curves of TiO2 and TiO2-N. | |
The TGA of TiO2 and TiO2-N were carried out to determine the extent of surface modification. As shown in Fig. 1d, before 400 ℃, the weight loss of the unmodified TiO2 is 2.57%, then remain stable until the temperature raised to 800 ℃. The loss is may due to the absorbed water and —OH groups on the surface of TiO2. TiO2-N nanoparticles displayed an obvious decomposition step at around 220 ℃ with a weight loss of 5.72%, which corresponds to the decomposition of the modifier APTMS.
Then we employed the prepared TiO2-N (x) nanoparticles as emulsifier to prepare Pickering emulsions, and investigated the influence of grafting amount of aminoproply groups on the property of Pickering emulsions. After stirring a mixture of toluene (4 mL), water (4 mL) and nanoparticles (0.8 wt% with respect to water) for 5–10 min, different phenomena appeared for TiO2 and TiO2-N (x). As shown in Fig. 2, for TiO2, the system consisted of two phases (oil and water), the TiO2 nanoparticles were all dispersed in water (the lower layer), none liquid drops could be observed in the optical micrograph. For the modified TiO2 nanoparticles, TiO2-N (x), Pickering emulsion phase formed at the upper layer and spherical droplets with diameters of 100–250 μm were clearly observed in the corresponding optical microscopy images. The drop test confirms that they were of oil in water (O/W) type. After adding a few drops of HCl solution (1 mol/L), there was no obvious change in the system with TiO2. Whereas, for the other four TiO2-N (x) particles stabilized Pickering emulsion systems, the emulsions were demulsified, as showed in the optical micrographs. Interestingly, when we added a few drops of NaOH solution (1 mol/L) and adjusted the pH to 7–8, the Pickering emulsions were formed again. The different behaviour of TiO2 nanoparticles before and after modification is attributed to the different surface properties. TiO2 nanopartiles are too hydrophilic to be stabilizer for Pickering emulsions due to the hydroxyl groups on their surface. For TiO2-N (x), the aminopropyl groups on the surface endow the material with moderate hydrophilicity and pH-responsibility. When HCl solution is added to the system, the amino groups on the surface of TiO2-N (x) are protonated, their surface wettability become too hydrophilic to stabilize Pickering emulsions.

|
Download:
|
| Fig. 2. Optical microscopy and appearance of 4 mL of toluene and 4 mL of water mixture in the presence 0.032 g of different nanoparticles (photographs taken after standing for 1 h). A: before adding HCl, B: pH is adjusted to 3–4 using HCl (1 mol/L); C: pH is adjusted to 7–8 using NaOH (1 mol/L). | |
However, in the experiment of cyclic test, we found that emulsification and demulsification process of TiO2-N (1.0) can be operated at 9 times (Fig. 3a), but for TiO2-N (0.5), TiO2-N (1.5) and TiO2-N (2.0), the process only can be operated at 2 or 3 cycles, implying that the amount of aminopropyl group on the surface of TiO2 nanoparticles will influence the responsive performance of the emulsions.
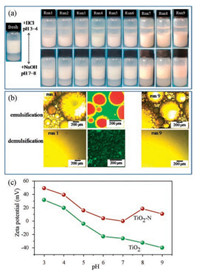
|
Download:
|
| Fig. 3. pH-triggered switch between emulsification and demulsification systems using 0.8 wt% (with respect to water) TiO2-N (1.0) particles. (a) Appearance of systems in 9 switch runs in response to pH. HCl solution (1 mol/L) and NaOH solution (1 mol/L) were used to adjust the pH. (b) Optical micrographs for the 1st and 9th run, and Laser scanning confocal microscopy images for the 1st run. Red represents the water phase and green is oil droplet. (c) Zeta potentials of TiO2 and TiO2-N in water at different pH values. | |
As expected, the emulsions stabilized by TiO2-N (x) are pHresponsive after acid protonates of the aminopropyl groups on the TiO2 surfaces. As Fig. 3a depicts, after adding a few drops of HCl solution to adjust the pH to 3–4 and stirring, the emulsions are destructed completely, and becoming a suspension in the lower layer. More interestingly, a Pickering emulsion system is regenerated from the suspension after a few drops NaOH solution are added and the pH are tuned back to 7–8 with stirring. Such a pH-triggered switch can be reversibly repeated at least 9 times. The emulsifying ability shows no significant change in each run. The optical micrographs confirm these results above (Fig. 3b). In the first run, the emulsion droplets of diameter about 80–250 μm are observed clearly. When pH decreases, these droplets disappear. The corresponding laser scanning confocal microscopy images further demonstrate formation and deformation of the Pickering emulsions. Green represents the water phase and red is oil droplet. The image confirms that Pickering emulsion phase is of O/W type. The same situation can be observed in the 9th run.
Such a pH-responsive behavior can be explained by the zeta potential. As shown in Fig. 3c, zeta potential of pure TiO2 nanoparticles is +31.9 mV at pH 3, with the increase of pH, the zeta potential gradually decreased to -26.2 mV at pH 7 owing to the deprotonation of hydroxyl groups on the surface of TiO2. While for TiO2-N, the zeta potential is +49.2 mV at pH 3, indicating the protonation of the surface aminopropyl groups. When the pH increases to 7, the zeta potential is approximately zero, suggesting a nearly complete deprotonation of the protonated aminopropyl. The difference between pure TiO2 and modified TiO2 lies in the pH-responsive surface chemistry.
To further confirm the ability of TiO2-N as a stabilizer for pH-responsive Pickering emulsions, the other organic solvents namely cyclohexane and benzene were used as the oil phase to prepare Pickering emulsions at a TiO2-N (1.0) concentration of 0.8 wt% (Fig. S1 in Supporting information). As shown in Fig. S1a, the Pickering emulsions can be formed with different oil phase, and the emulsions have pH-responsive property as water-toluene system. After adding HCl, the emulsions are breaking, while the emulsions reformed after NaOH being added. The pH-triggered switch can be reversibly repeated at 7 times and 4 times for watercyclohexane and water-benzene system, respectively (Figs. S1b and c). From the investigation result above, we can conclude that the TiO2-N particles can be used as an excellent recyclable pH-responsive Pickering emulsifier.
In order to investigate the catalytic efficiency of the pH switched Pickering emulsion system, we prepared a Pd/TiO2-N catalyst by loading Pd nanoparticles on TiO2-N (1.0) (for details see supporting information). As shown in Fig. S2 (Supporting information), the Pd nanoparticles (~1 nm in size) were uniformly distributed on the surface of TiO2-N.
The hydrogenation of styrene was chosen as a model reaction to evaluate the catalytic recyclability (see Supporting information for details). As expected, the Pickering emulsion system can be switch reversibly through adjusting the pH value (Fig. 4a). When the pH of the reaction system was adjusted to 3–4 at the end of reaction, the Pickering emulsion was demulsified under gentle stirring. The product in the organic phase can be separated by simple liquid transfer. The catalyst in the water phase could be directly used in the next batch reaction without any treatments. Pickering emulsion was formed again by adjusting the pH to 7–8 in the subsequent reaction cycles. As shown in Fig. 4b, from the second to sixth cycle, the conversions of styrene are more than 95%. From the seventh to the tenth reaction cycle, pH responsive property of the systems could be still existed, and conversions of between 78% and 88% were obtained. In the 10th reaction cycle, the droplet size had no significant change in comparison with those of the first reaction cycle (Fig. 4a). The loss of reaction efficiency may be mainly due to the Pd particle aggregation and slight Pd leaching. In the 10 reaction cycles, through only adjusting the pH values of the reaction system, the in situ separation of product and recycling of catalyst could be achieved.

|
Download:
|
| Fig. 4. (a) The appearance and corresponding microscopy images of the reaction system of the Pd/TiO2-N stabilized Pickering emulsion in the first reaction cycle and the tenth reaction cycle, respectively. (b) Recycling results of the hydrogenation of styrene over Pd/TiO2-N in the Pickering emulsion systems. Reaction conditions: styrene (3.0 mmol), toluene (4 mL), water (4 mL), Pd/TiO2-N (0.032 g), 40 ℃, 1 MPa, 120 min. | |
We further examined the catalytic activity of Pd/TiO2-N for hydrogenation of several other olefins in Pickering emulsion system. The reaction results are summarized in Table 1. For all the investigated substrates, more than 87% of conversions could be obtained in Pickering emulsion system. The excellent catalytic performance can be attributed to the lager reaction interface area in the Pickering emulsion system [39, 40].
|
|
Table 1 Results of hydrogenation of different substrates over Pd/TiO2-N.a |
In conclusion, a pH-responsive Pickering emulsion system was developed through simple surface modification of TiO2 nanoparticles. After loading Pd nanoparticles, the interficially active catalyst can be used in the hydrogenation of olefins. Apart from their excellent activity, the catalyst nanoparticles are readily recycled only by lower the pH and exhibit very good stability over repetitive emulsification–demulsification cycling (10 cycles).
AcknowledgmentsWe acknowledge the Natural Science Foundation of China (Nos. 21733009, 21573136, and U1510105), the Key Scientist and Technology Program of Shanxi Province (No. 20150313003-1), and Shanxi Scholarship Council of China (No. 2015-003).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.01.010.
| [1] |
F. Zaera, Chem. Soc. Rev. 42 (2013) 2746-2762. DOI:10.1039/C2CS35261C |
| [2] |
J. Grunes, J. Zhu, G.A. Somorjai, Chem. Commun. (2003), 2257-2260. |
| [3] |
Y.J. Xiong, B.J. Wiley, Y. Xia, Angew. Chem. Int. Ed. 46 (2007) 7157-7159. DOI:10.1002/(ISSN)1521-3773 |
| [4] |
R. Narayanan, M.A. El-Sayed, J. Phys. Chem. B 109 (2005) 12663-12676. DOI:10.1021/jp051066p |
| [5] |
R.J. White, R. Luque, V. Budarin, J.H. Clark, D.J. Macquarrie, Chem. Soc. Rev. 38 (2009) 481-494. DOI:10.1039/B802654H |
| [6] |
V. Polshettiwar, R.S. Varma, Green Chem. 12 (2010) 743-754. DOI:10.1039/b921171c |
| [7] |
X. Yang, D. Chen, S.J. Liao, et al., J. Catal. 291 (2012) 36-43. DOI:10.1016/j.jcat.2012.04.003 |
| [8] |
A.T. Bell, Science 299 (2003) 1688-1691. DOI:10.1126/science.1083671 |
| [9] |
C.W. Lim, I.S. Lee, Nano Today 5 (2010) 412-434. DOI:10.1016/j.nantod.2010.08.008 |
| [10] |
S. Shylesh, V. Schünemann, W.R. Thiel, Angew. Chem. Int. Ed. 49 (2010) 3428-3459. DOI:10.1002/anie.200905684 |
| [11] |
X.H. Li, W.L. Zheng, H.Y. Pan, et al., J. Catal. 300 (2013) 9-19. DOI:10.1016/j.jcat.2012.12.007 |
| [12] |
D.J. Cole-Hamilton, Science 299 (2003) 1702-1706. DOI:10.1126/science.1081881 |
| [13] |
Y.S. Lin, N. Abadeer, K.R. Hurley, C.L. Haynes, J. Am. Chem. Soc. 133 (2011) 20444-20457. DOI:10.1021/ja208567v |
| [14] |
N. Zhao, W. Ma, Z.M. Cui, et al., ACS Nano 3 (2009) 1775-1780. DOI:10.1021/nn900364g |
| [15] |
F.G. de Rivera, I. Angurell, M.D. Rossell, et al., Chem. Eur. J. 19 (2013) 11963-11974. DOI:10.1002/chem.201301769 |
| [16] |
E. Weiss, B. Dutta, Y. Schnell, Y. Schnell, R. Abu-Reziq, J. Mater. Chem. A 2 (2014) 3971-3977. DOI:10.1039/C3TA14992G |
| [17] |
Y. Zhu, L. Tian, Z. Jiang, et al., J. Catal. 281 (2011) 106-118. DOI:10.1016/j.jcat.2011.04.007 |
| [18] |
C.O. Dálaigh, S.A. Corr, Y. Gfflnko, S.J. Connon, Angew. Chem. Int. Ed. 46 (2007) 4329-4332. |
| [19] |
J.P. Huang, H.Q. Yang, Chem. Commun. 51 (2015) 7333-7336. DOI:10.1039/C5CC01211B |
| [20] |
H.Q. Yang, T. Zhou, W.J. Zhang, Angew. Chem. Int. Ed. 52 (2013) 7455-7459. DOI:10.1002/anie.201300534 |
| [21] |
Z.P. Wang, F.P.J.T. Rutjes, J.C.M. van Hest, Chem. Commun. 50 (2014) 14550-14553. DOI:10.1039/C4CC07048H |
| [22] |
B.T. Nguyen, W. Wang, B.R. Saunders, L. Benyahia, T. Nicolai, Langmuir 31 (2015) 3605-3611. DOI:10.1021/la5049024 |
| [23] |
J. Tang, P.J. Quinlan, K.C. Tam, Soft Matter 11 (2015) 3512-3529. DOI:10.1039/C5SM00247H |
| [24] |
S.S. Chen, Y.H. Zhu, W. Li, et al., Chin. J. Catal. 31 (2010) 605-614. DOI:10.1016/S1872-2067(09)60073-5 |
| [25] |
Y.L. Sui, S.B. Liu, T.F. Li, et al., J. Catal. 353 (2017) 250-255. DOI:10.1016/j.jcat.2017.07.024 |
| [26] |
K. Panwar, M. Jassal, A.K. Agrawal, RSC Adv. 6 (2016) 92754-92764. DOI:10.1039/C6RA12378C |
| [27] |
M. Hussain, M. Ahmad, A. Nisar, et al., New J. Chem. 38 (2014) 1424-1432. DOI:10.1039/c3nj01525d |
| [28] |
L. Liu, J.H. Kou, D.M. Guo, et al., Chin. Chem. Lett. 20 (2009) 1366-1370. DOI:10.1016/j.cclet.2009.06.026 |
| [29] |
M. Kacem, G. Plantard, N. Wery, V. Goetz, Chin. J. Catal. 35 (2014) 1571-1577. DOI:10.1016/S1872-2067(14)60212-6 |
| [30] |
N. Li, X.J. Lang, W.H. Ma, et al., Chem. Commun. 49 (2013) 5034-5036. DOI:10.1039/c3cc41407h |
| [31] |
Y. Kuwahara, K. Maki, Y. Matsumura, et al., J. Phys. Chem. C 113 (2009) 1552-1559. |
| [32] |
J.G. Yu, L.F. Qi, M. Jaroniec, J. Phys. Chem. C 114 (2010) 13118-13125. DOI:10.1021/jp104488b |
| [33] |
G.M. Wang, H.Y. Wang, Y.C. Ling, et al., Nano Lett. 11 (2011) 3026-3033. DOI:10.1021/nl201766h |
| [34] |
K.L. Chen, S.X. Zhou, RSC Adv. 5 (2015) 13850-13856. DOI:10.1039/C4RA16275G |
| [35] |
T. Chen, P.J. Colver, S.A.F. Bon, Adv. Mater. 19 (2007) 2286-2289. DOI:10.1002/(ISSN)1521-4095 |
| [36] |
Q. Zhang, R.X. Bai, T. Guo, T. Meng, ACS Appl. Mater. Interfaces 7 (2015) 18240-18246. DOI:10.1021/acsami.5b06808 |
| [37] |
M.F. Nsib, A. Maayoufi, N. Moussa, et al., J. Photochem. Photobiol. A:Chem. 251 (2013) 10-17. DOI:10.1016/j.jphotochem.2012.10.007 |
| [38] |
R.X. Bai, L.H. Xue, R.K. Dou, et al., Langmuir 32 (2016) 9254-9264. DOI:10.1021/acs.langmuir.6b02329 |
| [39] |
H.F. Liu, Z.M. Zhang, H.Q. Yang, F.Q. Cheng, Z.P. Du, ChemSusChem 7 (2014) 1888-1900. DOI:10.1002/cssc.v7.7 |
| [40] |
Y.H. Yu, L.M. Fu, F.W. Zhang, T. Zhou, H.Q. Yang, ChemPhysChem 15 (2014) 841-848. DOI:10.1002/cphc.v15.5 |
 2018, Vol. 29
2018, Vol. 29 



